Quality-Adjusted Time Without Symptoms of Disease or Toxicity (Q-TWiST) in Patients With Newly Diagnosed Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Comparison of Ponatinib Versus Imatinib
- PMID: 40165400
- PMCID: PMC11958597
- DOI: 10.1002/cam4.70780
Quality-Adjusted Time Without Symptoms of Disease or Toxicity (Q-TWiST) in Patients With Newly Diagnosed Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Comparison of Ponatinib Versus Imatinib
Abstract
Background: In the phase 3 ponatinib-3001 trial (PhALLCON, NCT03589326), ponatinib demonstrated superior efficacy over imatinib with comparable safety in patients with newly diagnosed Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). This post hoc analysis evaluated the net benefits of ponatinib using a quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) approach.
Methods: Overall survival (OS) time for patients from PhALLCON was partitioned into three health states: TOX (time with grade 3+ treatment-emergent adverse events [TEAEs] before disease progression), TWiST (time without toxicity before progression), and REL (time from progression until death or end of follow-up). Q-TWiST was calculated as the sum of health utility-weighted restricted mean durations of the three states. A relative Q-TWiST gain of ≥ 10% was considered clinically important. Sensitivity analyses were conducted by varying TOX and REL utilities, follow-up time, and the TOX definition (using grade 2+ TEAEs or patient-perceived treatment tolerability assessed by the FACT-GP5).
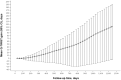
Results: Among all randomized patients (ponatinib n = 164, imatinib n = 81), restricted mean OS was similar between arms (1082.2 vs. 1024.8 days; p = 0.373). In the base-case analysis, mean TWiST was 214.5 days longer with ponatinib versus imatinib (95% CI 70.3-358.7; p = 0.004), REL was shorter by 175.9 days (325.4-26.5; p = 0.021), and TOX was not significantly different between arms (p = 0.228). The relative Q-TWiST gain (10.98%) was clinically important. Sensitivity analyses consistently supported the robustness of the base-case findings.
Conclusion: Ponatinib may prolong quality-adjusted survival compared with imatinib, supporting the benefit-risk profile of ponatinib as a front-line treatment for Ph+ ALL.
Trial registration: NCT03589326.
Keywords: Philadelphia chromosome‐positive; Q‐TWiST analysis; acute lymphoblastic leukemia; imatinib; ponatinib; quality of life; tyrosine kinase inhibitor.
© 2025 Takeda Pharmaceuticals USA, Inc. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Ajibade Ashaye and Deepali Rane report employment with Takeda. Ling Shi and Shien Guo report employment with Evidera, which received payment for the statistical analysis during the conduct of the study. Ibrahim Aldoss reports honoraria from Takeda, Amgen, Pfizer, Jazz, and Sobi; consulting/advisory roles at Takeda, Amgen, Pfizer, Jazz, and Sobi; participation in the speakers bureau at Pfizer; and research funding from AbbVie and MacroGenics. Pau Montesinos reports consulting or advisory roles at Takeda, Daiichi Sankyo, and Bristol Myers Squibb; participation in the speakers bureau at Servier; and research funding from AbbVie, Takeda, Daiichi Sankyo, Novartis, and Servier. Pankit Vachhani reports consulting/advisory roles at Blueprint Medicines, AbbVie, Amgen, Cogent Biosciences, Incyte, CTI BioPharma, Daiichi Sankyo, GlaxoSmithKline, Karyopharm, Novartis, Pfizer, Genentech, Servier, Stemline, and Morphosys. Vanderson Rocha reports consulting/advisory roles at Pfizer, Takeda, AbbVie, Astellas, Kite, and Amgen. Cristina Papayannidis reports consulting/advisory roles at AbbVie, Astellas, Novartis, Bristol Myers Squibb, Menarini, Stemline, Blueprint Medicines, Incyte, GlaxoSmithKline, Amgen, Pfizer, and Janssen; and personal fees from Astellas, Novartis, Pfizer, and Amgen. Jessica T. Leonard reports consulting/advisory roles at Adaptive Biotechnologies, Kite/Gilead, Pfizer, and Takeda; and support for travel, accommodation, and expenses from Adaptive Biotechnologies. Jose‐Maria Ribera reports consulting/advisory roles at Incyte, Pfizer, Bristol Myers Squibb, Novartis, and Takeda; and research funding from Amgen. James McCloskey reports personal fees from Bristol Myers Squibb, Takeda, Blueprint Medicine, PharmaEssentia, CTI, GlaxoSmithKline, Incyte, Amgen, and Jazz Pharmaceuticals. Jianxiang Wang reports an advisory role at Abbvie. Maria R. Baer has no conflicts of interest to disclose.
Figures


Similar articles
-
Patient-reported outcomes in Philadelphia chromosome-positive acute lymphoblastic leukemia patients treated with ponatinib or imatinib: results from the PhALLCON trial.Leukemia. 2025 Jun;39(6):1342-1350. doi: 10.1038/s41375-025-02608-4. Epub 2025 Apr 16. Leukemia. 2025. PMID: 40240572 Free PMC article. Clinical Trial.
-
Real-world outcomes in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia or chronic myeloid leukemia treated with ponatinib - final 6-year results from a Belgian registry.Hematology. 2025 Dec;30(1):2534196. doi: 10.1080/16078454.2025.2534196. Epub 2025 Aug 14. Hematology. 2025. PMID: 40811042
-
Comparison of third-generation tyrosine kinase inhibitor (TKI) ponatinib with first- and second-generation TKIs for treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia: A systematic review and bias-corrected meta-analysis.Crit Rev Oncol Hematol. 2025 Sep;213:104806. doi: 10.1016/j.critrevonc.2025.104806. Epub 2025 Jun 13. Crit Rev Oncol Hematol. 2025. PMID: 40517974 Review.
-
Ponatinib vs Imatinib in Frontline Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Randomized Clinical Trial.JAMA. 2024 Jun 4;331(21):1814-1823. doi: 10.1001/jama.2024.4783. JAMA. 2024. PMID: 38722621 Free PMC article. Clinical Trial.
-
Comparative Effectiveness of Newer Tyrosine Kinase Inhibitors Versus Imatinib in the First-Line Treatment of Chronic-Phase Chronic Myeloid Leukemia Across Risk Groups: A Systematic Review and Meta-Analysis of Eight Randomized Trials.Clin Lymphoma Myeloma Leuk. 2016 Jun;16(6):e85-94. doi: 10.1016/j.clml.2016.03.003. Epub 2016 Mar 30. Clin Lymphoma Myeloma Leuk. 2016. PMID: 27101984
References
-
- Fielding A. K., “Treatment of Philadelphia Chromosome‐Positive Acute Lymphoblastic Leukemia in Adults: A Broader Range of Options, Improved Outcomes, and More Therapeutic Dilemmas,” American Society of Clinical Oncology Educational Book 35, no. 1 (2015): e352, 10.14694/EdBook_AM.2015.35.e352. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

