The diagnostic role of pharmacological provocation testing in cardiac electrophysiology: a clinical consensus statement of the European Heart Rhythm Association and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC, the ESC Working Group on Cardiovascular Pharmacotherapy, the Association of European Paediatric and Congenital Cardiology (AEPC), the Paediatric & Congenital Electrophysiology Society (PACES), the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS)
- PMID: 40165484
- PMCID: PMC12018878
- DOI: 10.1093/europace/euaf067
The diagnostic role of pharmacological provocation testing in cardiac electrophysiology: a clinical consensus statement of the European Heart Rhythm Association and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC, the ESC Working Group on Cardiovascular Pharmacotherapy, the Association of European Paediatric and Congenital Cardiology (AEPC), the Paediatric & Congenital Electrophysiology Society (PACES), the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS)
Abstract
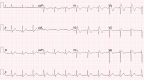
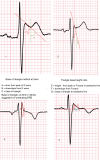
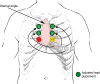
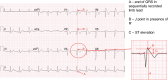
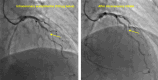
The pharmacological provocation test is a pivotal tool in cardiac electrophysiology for the diagnosis of potential causes of sudden cardiac death, sudden cardiac arrest (SCA), arrhythmias, symptoms, or ECG abnormalities. The 2022 European Society of Cardiology Guidelines for the Treatment of Ventricular Arrhythmias and Prevention of Sudden Cardiac Death offered guidance on provocation testing but did not describe the indications and requirements in depth. This clinical consensus statement, led by the European Heart Rhythm Association and approved by major international stakeholders, aims to advise the general cardiologist and the arrhythmia expert who to test and when, where, and how to do it. The statement focuses on current practice for the diagnosis of subclinical arrhythmia syndromes and the causes of SCA, building upon the recommendations of the Guidelines. We address the sodium channel blocker provocation test for patients suspected of Brugada syndrome as well as the use of epinephrine, isoproterenol, adenosine, ergonovine, and acetylcholine.
Keywords: Brugada syndrome; Wolff-Parkinson-White syndrome; acetylcholine; adenosine; ajmaline; cardiac arrest; catecholaminergic polymorphic ventricular tachycardia; coronary vasospasm; drug challenge; epinephrine; ergonovine; flecainide; isoproterenol; pilsicainide; procainamide; provocation testing; sodium channel blocker test; sudden arrhythmic death syndrome; sudden cardiac death.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: No conflicts of interest related to the document topic were declared. E.R.B. declares consulting fees for Boston Scientific and Solid Biosciences and speaker fees for Johnson and Johnson. E.A. declares consulting fees for Boston Scientific and speaker fees for Medtronic and Bristol Myer Squibb. B.G.W. declares consulting and speaker fees for Sanofi.
Figures


References
-
- Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. - PubMed
-
- Papadakis M, Papatheodorou E, Mellor G, Raju H, Bastiaenen R, Wijeyeratne Y et al. The diagnostic yield of Brugada syndrome after sudden death with normal autopsy. J Am Coll Cardiol 2018;71:1204–14. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

