The Efficacy, Safety and Longevity of Biologic Treatments in Pediatric and Adult Psoriasis Patients: A Comparative Multi-Center, Real-Life Study
- PMID: 40165569
- PMCID: PMC11965878
- DOI: 10.5021/ad.24.057
The Efficacy, Safety and Longevity of Biologic Treatments in Pediatric and Adult Psoriasis Patients: A Comparative Multi-Center, Real-Life Study
Abstract
Background: Evidence on the effectiveness, long-term safety and longevity of biologic therapies in pediatric psoriasis patients is sparse.
Objective: This study aims to compares the efficacy, safety and drug survival (DS) rates of etanercept (ETA), adalimumab (ADA), infliximab (INF), ustekinumab (UST), secukinumab (SEC) and ixekizumab (IXE) in pediatric and adult psoriasis patients.
Methods: 293 biologic treatment cycles of 198 patients (62 pediatric and 136 adult) from three academic psoriasis referral centres were analysed.
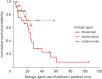
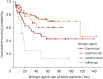
Results: The following were the Psoriasis Area and Severity Index 90 response scores of pediatric and adult psoriasis patients, respectively: ETA, 42.3% vs. 34.6%; ADA, 53.8% vs. 59.8%; INF, 33.3% vs. 33.3%; UST, 76.5% vs. 56.8%; SEC, 60% vs. 60%; and IXE, 50% vs. 87.5%. The differences of responses between the two groups were statistically insignificant (p>0.05). ETA had the longest mean DS time in the pediatric group but it was related to a significantly shorter DS in pediatric patients than in adults (pediatrics: 30.58 [18.64-42.52] months vs. adults: 72.34 [54.70-89.99] months; p=0.025). ADA had the longest mean DS time in the adult group with 101.28 [84.88-117.68] months. All treatments had favorable safety profiles. No specific severe adverse effects necessitating treatment discontinuation were observed in pediatric patients.
Conclusion: Although responses to ETA and UST were numerically better among children, the difference was insignificant. The DS rates in each group were comparable, and no specific safety signals, limiting the long-term use of these agents, were detected in the pediatric group.
Keywords: Biologics; Psoriasis; Skin diseases; Treatment efficacy.
© 2025 The Korean Dermatological Association and The Korean Society for Investigative Dermatology.
Conflict of interest statement
The authors have nothing to disclose.
Figures
References
-
- Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537–1541. - PubMed
-
- Burden-Teh E, Thomas KS, Ratib S, Grindlay D, Adaji E, Murphy R. The epidemiology of childhood psoriasis: a scoping review. Br J Dermatol. 2016;174:1242–1257. - PubMed
-
- Cvenkel K, Starbek Zorko M. Challenges in the treatment of psoriasis in childhood. Acta Dermatovenerol Alp Panonica Adriat. 2021;30:105–108. - PubMed
-
- Menter A, Cordoro KM, Davis DMR, Kroshinsky D, Paller AS, Armstrong AW, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161–201. - PubMed
-
- Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029–1072. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials