New potent N-hydroxycinnamamide-based histone deacetylase inhibitors suppress proliferation and trigger apoptosis in THP-1 leukaemia cells
- PMID: 40165669
- PMCID: PMC11959351
- DOI: 10.1002/ardp.202400889
New potent N-hydroxycinnamamide-based histone deacetylase inhibitors suppress proliferation and trigger apoptosis in THP-1 leukaemia cells
Abstract
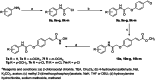
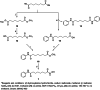
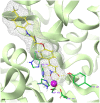
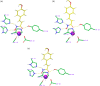
A new group of potent histone deacetylase inhibitors (HDACis) capable of inhibiting cell growth and affecting cell-cycle progression in Tohoku Hospital Pediatrics-1 (THP-1) monocytic leukaemia cells was synthesized. The inhibitors belong to a series of hydroxamic acid derivatives. We designed and synthesized a series of 22 N-hydroxycinnamamide derivatives, out of which 20 are new compounds. These compounds contain various substituted anilides as the surface recognition moiety (SRM), a p-hydroxycinnamate linker, and hydroxamic acids as the zinc-binding group (ZBG). The whole series of synthesized hydroxamic acids inhibited THP-1 cell proliferation. Compounds 7d and 7p, which belong to the category of derivatives with the most potent antiproliferative properties, exert a similar effect on cell-cycle progression as vorinostat and induce apoptosis in THP-1 cells. Furthermore, compounds 7d and 7p were demonstrated to inhibit HDAC class I and II in THP-1 cells with comparable potency to vorinostat and increase acetylation of histones H2a, H2b, H3, and H4. Molecular modelling was used to predict the probable binding mode of the studied HDACis in class I and II histone deacetylases in terms of Zn2+ ion chelation by the hydroxamate group.
Keywords: HDACi; anticancer agents; haematological malignancies; hydroxamic acid; inhibitors of histone deacetylases.
© 2025 The Author(s). Archiv der Pharmazie published by Wiley‐VCH GmbH on behalf of Deutsche Pharmazeutische Gesellschaft.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

