A Prospective, Multicenter Analysis of Recurrence-Free Survival After Sentinel Lymph Node Biopsy Decisions Influenced by the 31-GEP
- PMID: 40165762
- PMCID: PMC11959192
- DOI: 10.1002/cam4.70839
A Prospective, Multicenter Analysis of Recurrence-Free Survival After Sentinel Lymph Node Biopsy Decisions Influenced by the 31-GEP
Abstract
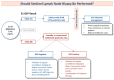
Background: Although most patients with cutaneous melanoma (CM) will have a negative sentinel lymph node biopsy (SLNB), up to 20%-30% of these patients will recur. The 31-gene expression profile (31-GEP) test has been prospectively validated to identify patients at low (Class 1A), intermediate (Class 1B/2A), and high (Class 2B) risk of SLN positivity and recurrence.
Methods: DECIDE is a prospective, multicenter study to assess the effect of 31-GEP testing on SLNB performance rates in patients with T1-T2 tumors considering SLNB and to study long-term outcomes. Here, we assessed outcomes in patients with a Class 1A 31-GEP result (n = 130).
Results: Of Class 1A patients, 63 had an SLNB, with a 3.2% SLN positivity rate (2/63). No Class 1A patients, regardless of SLN status, experienced a recurrence (2-year median follow-up).
Conclusions: These results are consistent with previous studies that showed the 31-GEP can identify patients at low risk of SLN positivity and recurrence.
© 2025 Castle Biosciences Inc and The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
This study was funded by Castle Biosciences Inc. B.J.M. is an employee and stock/options holder at Castle Biosciences Inc. J.M.G. and R.S. are on the Speaker's bureau for Castle Biosciences. Additional authors have no conflicts to declare.
Figures
References
-
- Swetter S. M., Johnson D., Thompson J. A., et al., “NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Melanoma: Cutaneous V.3.2023,” © National Comprehensive Cancer Network, Inc. (2023), https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf.
-
- Moody J. A., Ali R. F., Carbone A. C., Singh S., and Hardwicke J. T., “Complications of Sentinel Lymph Node Biopsy for Melanoma – A Systematic Review of the Literature,” European Journal of Surgical Oncology 43, no. 2 (2017): 270–277. - PubMed
-
- Thomas D. C., Han G., Leong S. P., et al., “Recurrence of Melanoma After a Negative Sentinel Node Biopsy: Predictors and Impact of Recurrence Site on Survival,” Annals of Surgical Oncology 26, no. 7 (2019): 2254–2262. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical