Effect of gaseous ozone treatment on cells and biofilm of dairy Bacillus spp. isolates
- PMID: 40165788
- PMCID: PMC11955631
- DOI: 10.3389/fmicb.2025.1538456
Effect of gaseous ozone treatment on cells and biofilm of dairy Bacillus spp. isolates
Abstract
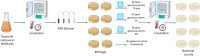
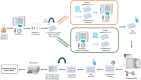
Bacillus spp. can produce biofilms and cause recurrent contamination in the food industry. The common clean-in-place (CIP) method is usually employed in sanitizing processing equipment. However, CIP is not always effective in removing biofilms. Ozone represents a promising "green" alternative to control biofilms. In this study, the effect of gaseous ozone (50 ppm) was evaluated in vitro against planktonic and sessile B. cereus and B. subtilis isolates collected from the dairy sector. Planktonic cells were enumerated by plate counts after 10 min, 1 h, and 6 h of ozone treatment. After a short-term (10 min) exposure, a slight reduction in microbial loads (0.66-2.27 ± 0.15 Log10 CFU/mL) was observed for B. cereus strains, whereas a more pronounced reduction (2.90-3.81 ± 0.12 Log10 CFU/mL) was noted in B. subtilis isolates. The microbial load further decreased after 1 h-treatments, around 1.5-3.46 ± 0.11 Log10 CFU/mL for B. cereus strains, and 4.0-5.6 ± 0.11 Log10 CFU/mL for B. subtilis isolates, until complete inactivation of bacterial cells after 6 h of exposure. Moreover, the effect of gaseous ozone treatment (50 ppm, 6 h) was evaluated for its ability to inhibit and eradicate biofilms formed on two common food-contact materials (polystyrene and stainless steel). Sessile B. subtilis cells were the more sensitive to the action of ozone, while a weak effect was highlighted on B. cereus isolates on both surface types. These results were further confirmed by scanning microscopy analysis. The number of cells in the biofilm state was also assessed, showing a not-complete correlation with a decrease in Biofilm Production Indices (BPIs). These findings highlighted the effectiveness of the sanitizing protocol using gaseous ozone in contrasting Bacillus free-living cells, but a not completely counteraction in biofilm formation (inhibition) or eradication of pre-formed biofilm. Thus, the application of ozone could be thought of not alone, but in combination with common sanitization practices to improve their effectiveness.
Keywords: Bacillus cereus; Bacillus subtilis; antimicrobial biofilm; food contact surfaces; gaseous ozone.
Copyright © 2025 Catania, Dalmasso, Morra, Costa, Bottero and Di Ciccio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
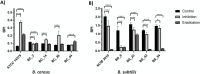
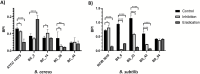


Similar articles
-
Evaluation of the biofilm-forming ability and molecular characterization of dairy Bacillus spp. isolates.Front Cell Infect Microbiol. 2023 Jul 31;13:1229460. doi: 10.3389/fcimb.2023.1229460. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37600945 Free PMC article.
-
Efficacy of gaseous chlorine dioxide in inactivating Bacillus cereus spores attached to and in a biofilm on stainless steel.Int J Food Microbiol. 2014 Oct 1;188:122-7. doi: 10.1016/j.ijfoodmicro.2014.07.009. Epub 2014 Jul 18. Int J Food Microbiol. 2014. PMID: 25090607
-
Effect of Gaseous Ozone on Listeria monocytogenes Planktonic Cells and Biofilm: An In Vitro Study.Foods. 2021 Jun 26;10(7):1484. doi: 10.3390/foods10071484. Foods. 2021. PMID: 34206833 Free PMC article.
-
The Use of Ozone as an Eco-Friendly Strategy against Microbial Biofilm in Dairy Manufacturing Plants: A Review.Microorganisms. 2022 Jan 13;10(1):162. doi: 10.3390/microorganisms10010162. Microorganisms. 2022. PMID: 35056612 Free PMC article. Review.
-
Dry surface biofilms in the food processing industry: An overview on surface characteristics, adhesion and biofilm formation, detection of biofilms, and dry sanitization methods.Compr Rev Food Sci Food Saf. 2023 Jan;22(1):688-713. doi: 10.1111/1541-4337.13089. Epub 2022 Dec 17. Compr Rev Food Sci Food Saf. 2023. PMID: 36464983 Review.
References
-
- Cai Y., Sun T., Li G., An T. (2021). Traditional and emerging water disinfection technologies challenging the control of antibiotic-resistant bacteria and antibiotic resistance genes. ACS EST Engg. 1, 1046–1064. doi: 10.1021/acsestengg.1c00110 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

