Combined standard immunosuppression and immune checkpoint inhibition for BKPyV+ metastatic renal cell carcinoma of the graft in a kidney transplant recipient with chronic rejection: a case report
- PMID: 40165886
- PMCID: PMC11955445
- DOI: 10.3389/fonc.2025.1506324
Combined standard immunosuppression and immune checkpoint inhibition for BKPyV+ metastatic renal cell carcinoma of the graft in a kidney transplant recipient with chronic rejection: a case report
Abstract
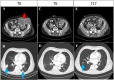
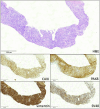
We report on the first case of a dual-kidney transplant recipient diagnosed with a metastatic BK polyomavirus-positive clear renal cell carcinoma with sarcomatoid features, which caused extensive vena cava thrombosis. The patient was successfully treated with the immune checkpoint inhibitors (ICIs) ipilimumab plus nivolumab and continued immunosuppression with tacrolimus, mycophenolate, and steroids. He received ICIs despite the presence of graft dysfunction due to transplant glomerulopathy. As expected, the ICI treatment caused a progressive but asymptomatic decline of the graft function, which resulted in end-stage kidney disease. However, continuation of a full immunosuppression prevented acute rejection, graft intolerance syndrome episodes, or dual graft nephrectomy, which enabled the patient to successfully continue ICIs while on dialysis and to achieve sustained partial remission at the 17-month follow-up.
Keywords: BK polyomavirus; dialysis; immune checkpoint inhibitors; immunosuppression; kidney transplantation; renal cell carcinoma.
Copyright © 2025 Gandolfini, Manini, Benigno, Gentile, Palmisano, Somenzi, Gnetti, Delsante, Mordà, D’Angelo, Salvetti, Fiaccadori, Buti and Maggiore.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Hellemans R, Pengel LHM, Choquet S, Maggiore U, for EWotTLJp . Managing immunosuppressive therapy in potentially cured post-kidney transplant cancer (excluding non-melanoma skin cancer): an overview of the available evidence and guidance for shared decision-making. Transpl Int. (2021) 34:1789–800. doi: 10.1111/tri.v34.10 - DOI - PMC - PubMed
-
- Tannir NM, Albiges L, McDermott DF, Burotto M, Choueiri TK, Hammers HJ, et al. . Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 8-year follow-up results of efficacy and safety from the phase III CheckMate 214 trial. Ann Oncol. (2024) 35:1026–38. doi: 10.1016/j.annonc.2024.07.727 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

