Suzetrigine, a Non-Opioid NaV1.8 Inhibitor With Broad Applicability for Moderate-to-Severe Acute Pain: A Phase 3 Single-Arm Study for Surgical or Non-Surgical Acute Pain
- PMID: 40165940
- PMCID: PMC11955400
- DOI: 10.2147/JPR.S509144
Suzetrigine, a Non-Opioid NaV1.8 Inhibitor With Broad Applicability for Moderate-to-Severe Acute Pain: A Phase 3 Single-Arm Study for Surgical or Non-Surgical Acute Pain
Abstract
Background: Many patients experience inadequate pain control due to limited options that are both efficacious and safe for treating moderate-to-severe acute pain; therefore, opioids are still frequently prescribed for their effectiveness despite known tolerability issues and safety concerns. Suzetrigine, an oral, non-opioid, offers a promising alternative by selectively inhibiting the voltage-gated sodium channel 1.8 (NaV1.8), a novel therapeutic target for pain management. Given the high selectivity of suzetrigine for NaV1.8 (does not bind to other sodium channels/receptors with CNS activity), suzetrigine does not have CNS side effects or addictive potential associated with opioids. In the largest randomized, controlled phase 3 trials in established acute pain models, suzetrigine monotherapy demonstrated statistically significant and clinically meaningful reduction in moderate-to-severe acute pain compared to placebo.
Methods: To evaluate the safety and effectiveness of suzetrigine for the treatment of moderate-to-severe-acute surgical and non-surgical pain conditions, we conducted a phase 3, single-arm study in adults with moderate or severe acute pain on the verbal categorical rating scale and ≥4 on the numeric pain rating scale following surgical procedures or after presenting to a medical facility with moderate or severe acute pain of new origin. Participants received suzetrigine (100mg then 50mg every 12hrs) for 14 days or pain resolution, whichever came first. The primary endpoint was safety. The secondary endpoint was participant perception of suzetrigine's effectiveness in treating acute pain at the end of treatment using a patient global assessment.
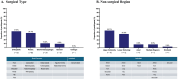
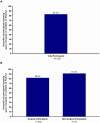
Results: Suzetrigine was generally safe and well-tolerated in participants (N=256) with a range of surgical and non-surgical acute pain conditions; the maximum severity for most participants who had adverse events was mild (71 participants; 27.7%) or moderate (21 participants; 8.2%). Most participants (213 participants; 83.2%) rated suzetrigine's effectiveness for treating pain on a patient global assessment as good, very good, or excellent.
Conclusion: Suzetrigine provides a safe and effective non-opioid, non-addictive treatment with broad applicability for moderate-to-severe acute pain.
Clinicaltrialsgov registration: NCT05661734.
Keywords: NaV1.8 inhibitor; VX-548; moderate-to-severe acute pain; non-opioid; surgical/nonsurgical acute pain; suzetrigine.
© 2025 McCoun et al.
Conflict of interest statement
Drs. McCoun, Solanki, Urban, Bertoch, Oswald, Swisher, Taber, and Weiner have reported personal fees from Vertex Pharmaceuticals as members of the Acute Pain Steering Committee. Dr. Urban has reported personal fees and honoraria from Pacira Biosciences. Dr. Oswald participates on a Data Safety Monitoring Board for Salix Pharmaceuticals. Dr. Swisher reports funding paid to his institution from SPR Therapeutics, Infutronix, and Avanos Medical, during the conduct of the study. Drs. Healey, Jazic, Correll, Negulescu, and Bozic are employees of Vertex Pharmaceuticals and own stock and/or options in the company. Dr. Weiner reports personal fees from Cessation Therapeutics, Inc., outside the submitted work. The authors report no other conflicts of interest in this work.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

