Mucosal multivalent NDV-based vaccine provides cross-reactive immune responses against SARS-CoV-2 variants in animal models
- PMID: 40165947
- PMCID: PMC11955676
- DOI: 10.3389/fimmu.2025.1524477
Mucosal multivalent NDV-based vaccine provides cross-reactive immune responses against SARS-CoV-2 variants in animal models
Abstract
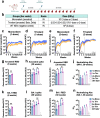
Introduction: A new generation of mucosal vaccine against the ever-evolving SARS-CoV-2 is of great value to fight COVID-19. In previous studies, our groups developed a viral vector vaccine based on an avirulent Newcastle disease virus (NDV) expressing the prefusion-stabilized spike protein of SARS-CoV-2 (NDV-HXP-S).
Methods: Here we characterized the in vivo biodistribution and immunogenicity of a live mucosal NDV-HXP-S vaccine in animal models.
Results: NDV showed restricted replication in mice and hamsters. Despite limited replication, intranasal live NDV-HXP-S provided protection against SARS-CoV-2 challenge and direct-contact transmission in hamsters. Importantly, a trivalent live NDV-HXP-S vaccine (Wuhan, Beta, Delta) induced more cross-reactive antibody responses against the phylogenetically distant Omicron variant than the ancestral vaccine. Furthermore, intranasal trivalent live NDV-HXP-S boosted systemic and mucosal immunity in mice pre-immunized with mRNA vaccine.
Discussion: Overall, a mucosal multivalent live NDV-HXP-S vaccine shows great promise as a safe, next-generation vaccine conferring broad mucosal and systemic immunity against future SARS-CoV-2 variants.
Keywords: COVID-19; Coronavirus; sterilizing immunity; transmission-proof; vector vaccine; viral shedding.
Copyright © 2025 González-Domínguez, Abdeljawad, Lai, Boza, McCroskery, Lemus, Slamanig, Singh, Warang, Yellin, Abbad, Carreño, Dolange, Martínez-Guevara, Singh, Barcena-Varela, Chang, Schotsaert, Krammer, Palese and Sun.
Conflict of interest statement
The Icahn School of Medicine at Mount Sinai has filed patent applications entitled “RECOMBINANT NEWCASTLE DISEASE VIRUS EXPRESSING SARS-COV-2 SPIKE PROTEIN AND USES THEREOF” which names IG-D, PP, FK and WS as inventors. Mount Sinai is seeking to commercialize this vaccine; therefore, the institution and its faculty inventors could benefit financially. Mount Sinai has spun out a company, CastleVax to commercialize the NDV-based SARS-CoV.2 vaccine. PP, FK and WS serve on the scientific advisory board of CastleVax and are listed as co-founders of the company. FK has consulted for Merck, Seqirus, Curevac and Pfizer, and is currently consulting for Pfizer, Third Rock Ventures, GSK and Avimex. The FK laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. The M.S. laboratory has received unrelated research funding in sponsored research agreements from 7Hills Pharma, ArgenX N.V., Moderna and Phio Pharmaceuticals, which has no competing interest with this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, et al. . Coronavirus pandemic (COVID-19) (2020). Available online at: https://ourworldindata.org/covid-vaccinationscitation (Accessed July 11, 2023).
-
- Lara-Puente JH, Carreno JM, Sun W, Suarez-Martinez A, Ramirez-Martinez L, Quezada-Monroy F, et al. . Safety and immunogenicity of a newcastle disease virus vector-based SARS-coV-2 vaccine candidate, AVX/COVID-12-HEXAPRO (Patria), in pigs. mBio. (2021) 12:e0190821. doi: 10.1128/mBio.01908-21 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

