Engineering a novel HSV-1 strain for oncolytic therapy of solid tumors
- PMID: 40165989
- PMCID: PMC11957669
- DOI: 10.1016/j.omton.2025.200961
Engineering a novel HSV-1 strain for oncolytic therapy of solid tumors
Abstract
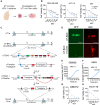
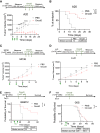
Oncolytic herpes simplex virus (HSV)-1-derived viruses are being developed for cancer treatment. Here, we describe the isolation of a novel strain of HSV-1 and its engineering to safely harness it as an oncolytic therapeutic. This strain (UT1a) was isolated from a de-identified consented patient biorepository. CRISPR-Cas9-based recombination was utilized to insert bacterial artificial chromosome (BAC) genes into the viral UL39 and UL40 locus, resulting in the deletion of both large and small subunits of the viral ribonucleotide reductase (RR). Subsequent deletion of viral RL1 genes encoding the neurovirulence factor γ34.5 resulted in OncoDelta (OncoD), a virus deleted for UL39, UL40, and both copies of RL1. OncoD retained tumor-cell-specific cytotoxicity and replication; was safe and non-toxic in intracranial injections in naive mice up to doses of 5 × 106, the maximal injectable dose for OncoD; and showed significant anti-tumor immune-activating potential in multiple tumor models. Transcriptome profiling of OncoD showed that it impaired DNA damage repair pathways and hence synergized with radiation to improve therapeutic response in vitro and in vivo.
Keywords: DNA damage; cancer therapy; herpes simplex virus; irradiation; oncolytic HSV-1; solid tumor.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Russell L., Swanner J., Jaime-Ramirez A.C., Wang Y., Sprague A., Banasavadi-Siddegowda Y., Yoo J.Y., Sizemore G.M., Kladney R., Zhang J., et al. PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance. Nat. Commun. 2018;9:5006. doi: 10.1038/s41467-018-07344-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
