This is a preprint.
Epigenomic subtypes of late-onset Alzheimer's disease reveal distinct microglial signatures
- PMID: 40166175
- PMCID: PMC11957029
- DOI: 10.1101/2025.03.15.643144
Epigenomic subtypes of late-onset Alzheimer's disease reveal distinct microglial signatures
Abstract
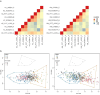
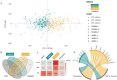
Growing evidence suggests that clinical, pathological, and genetic heterogeneity in late-onset Alzheimer's disease contributes to variable therapeutic outcomes, potentially explaining many trial failures. Advances in molecular subtyping through proteomic and transcriptomic profiling reveal distinct patient subgroups, highlighting disease complexity beyond amyloid-beta plaques and tau tangles. This insight underscores the need to expand molecular subtyping across new molecular layers, to identify novel drug targets for different patient subgroups. In this study, we analyzed genome-wide DNA methylation data from three independent postmortem brain cohorts (n = 831) to identify epigenetic subtypes of late-onset Alzheimer's disease. Unsupervised clustering approaches were employed to identify distinct DNA methylation patterns, with subsequent cross-cohort validation to ensure robustness and reproducibility. To explore the cell-type specificity of the identified epigenomic subtypes, we characterized their methylation signatures utilizing DNA methylation profiles derived from purified brain cells. Transcriptomic data from bulk and single-cell RNA sequencing were integrated to examine the functional impact of epigenetic subtypes on gene expression profiles. Finally, we performed statistical analyses to investigate associations between these DNA methylation-defined subtypes and clinical or neuropathological features, aiming to elucidate their biological significance and clinical implications. We identified two distinct epigenomic subtypes of late-onset Alzheimer's disease, each defined by reproducible DNA methylation patterns across three cohorts. Both subtypes exhibit cell-type-specific DNA methylation profiles. Subtype 1 and subtype 2 show significant microglial methylation enrichment, with odds ratios (OR) of 1.6 and 1.3, respectively. The minimal overlap between them suggests distinct microglial states. Additionally, subtype 2 displays strong neuronal (OR = 1.6) and oligodendrocyte (OR = 3.6) enrichment. Bulk transcriptomic analyses further highlighted divergent biological mechanisms underpinning these subtypes, with subtype 1 enriched for immune-related processes, and subtype 2 characterized predominantly by neuronal and synaptic functional pathways. Single-cell transcriptional profiling of microglia revealed subtype-specific inflammatory states: subtype 1 represented a state of chronic innate immune hyperactivation with impaired resolution, while subtype 2 exhibited a more dynamic inflammatory profile balancing pro-inflammatory signaling with reparative and regulatory mechanisms. This study highlights the molecular heterogeneity of late-onset Alzheimer's disease by identifying two epigenetic subtypes with distinct cell-type-specific DNA methylation patterns. Their alignment with previously defined molecular classifications underscores their relevance in disease pathogenesis. By linking these subtypes to inflammatory microglial activity, our findings provide a foundation for future precision medicine approaches in Alzheimer's research and treatment.
Keywords: Alzheimer’s disease; DNA methylation; Epigenetics; Inflammation; Microglia; Subtyping.
Conflict of interest statement
Competing interests The authors have no competing interests to declare.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
