This is a preprint.
ATP hydrolysis-driven structural transitions within the S. cerevisiae Rad51 and Dmc1 nucleoprotein filaments
- PMID: 40166259
- PMCID: PMC11957116
- DOI: 10.1101/2025.03.19.644215
ATP hydrolysis-driven structural transitions within the S. cerevisiae Rad51 and Dmc1 nucleoprotein filaments
Update in
-
ATP hydrolysis-driven structural transitions within the Saccharomyces cerevisiae Rad51 and Dmc1 nucleoprotein filaments.J Biol Chem. 2025 Jul 26;301(9):110528. doi: 10.1016/j.jbc.2025.110528. Online ahead of print. J Biol Chem. 2025. PMID: 40721016 Free PMC article.
Abstract
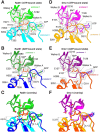
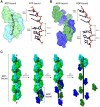
Homologous recombination (HR) is essential for the maintenance of genome stability and for generating genetic diversity during meiosis. The eukaryotic protein Rad51 is member of the Rad51/RecA family of DNA recombinases and is responsible for guiding the DNA pairing reactions that take place in HR during mitosis. Dmc1 is a meiosis-specific paralog of Rad51 and is responsible for the DNA pairing reactions that take place in HR during meiosis. Rad51 and Dmc1 are both ATP-dependent DNA-binding proteins and both form extended helical filaments on ssDNA which are key intermediates in HR. The stability of these nucleoprotein filaments is highly regulated and is also tightly coupled to nucleotide binding and hydrolysis. ATP binding promotes filament assembly whereas the hydrolysis of ATP to ADP reduces filament stability to promote filament disassembly. Here, we present CryoEM structures of the Saccharomyces cerevisiae recombinases Rad51 and Dmc1 in the ADP-bound states and provide a detailed structural comparison to the ATP-bound filaments. Our findings yield insights into the structural transitions that take place during the hydrolysis of ATP to ADP and suggest a new model for how these structural changes may be linked to nucleoprotein filament disassembly.
Figures




References
-
- Coop G. and Przeworski M., An evolutionary view of human recombination. Nat Rev Genet, 2007. 8(1): p. 23–34. - PubMed
-
- Ortiz-Barrientos D., Engelstadter J., and Rieseberg L.H., Recombination Rate Evolution and the Origin of Species. Trends Ecol Evol, 2016. 31(3): p. 226–36. - PubMed
-
- Cox M.M., et al. , The importance of repairing stalled replication forks. Nature, 2000. 404(6773): p. 37–41. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
