This is a preprint.
Decoupling Phase Separation and Fibrillization Preserves Activity of Biomolecular Condensates
- PMID: 40166274
- PMCID: PMC11957012
- DOI: 10.1101/2025.03.18.643977
Decoupling Phase Separation and Fibrillization Preserves Activity of Biomolecular Condensates
Abstract
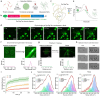
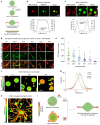
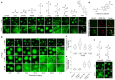
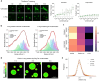
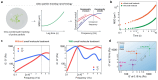
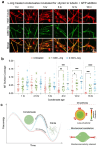
Age-dependent transition of metastable, liquid-like protein condensates to amyloid fibrils is an emergent phenomenon of numerous neurodegeneration-linked protein systems. A key question is whether the thermodynamic forces underlying reversible phase separation and maturation to irreversible amyloids are distinct and separable. Here, we address this question using an engineered version of the microtubule-associated protein Tau, which forms biochemically active condensates. Liquid-like Tau condensates exhibit rapid aging to amyloid fibrils under quiescent, cofactor-free conditions. Tau condensate interface promotes fibril nucleation, impairing their activity to recruit tubulin and catalyze microtubule assembly. Remarkably, a small molecule metabolite, L-arginine, selectively impedes condensate-to-fibril transition without perturbing phase separation in a valence and chemistry-specific manner. By heightening the fibril nucleation barrier, L-arginine counteracts age-dependent decline in the biochemical activity of Tau condensates. These results provide a proof-of-principle demonstration that small molecule metabolites can enhance the metastability of protein condensates against a liquid-to-amyloid transition, thereby preserving condensate function.
Conflict of interest statement
Competing interests P.R.B. is a member of the Biophysics Reviews (AIP Publishing) editorial board. This affiliation did not influence the work reported here. All other authors have no conflicts to report.
Figures
Similar articles
-
Decoupling Phase Separation and Fibrillization Preserves Activity of Biomolecular Condensates.Res Sq [Preprint]. 2025 Apr 29:rs.3.rs-6405673. doi: 10.21203/rs.3.rs-6405673/v1. Res Sq. 2025. PMID: 40343340 Free PMC article. Preprint.
-
The interface of condensates of the hnRNPA1 low-complexity domain promotes formation of amyloid fibrils.Nat Chem. 2023 Oct;15(10):1340-1349. doi: 10.1038/s41557-023-01289-9. Epub 2023 Sep 25. Nat Chem. 2023. PMID: 37749234 Free PMC article.
-
Tunable metastability of condensates reconciles their dual roles in amyloid fibril formation.bioRxiv [Preprint]. 2025 Mar 22:2024.02.28.582569. doi: 10.1101/2024.02.28.582569. bioRxiv. 2025. Update in: Mol Cell. 2025 Jun 5;85(11):2230-2245.e7. doi: 10.1016/j.molcel.2025.05.011. PMID: 38464104 Free PMC article. Updated. Preprint.
-
Liquid - liquid phase separation of tau: Driving forces, regulation, and biological implications.Neurobiol Dis. 2023 Jul;183:106167. doi: 10.1016/j.nbd.2023.106167. Epub 2023 May 23. Neurobiol Dis. 2023. PMID: 37230179 Review.
-
Liquid-liquid phase separation of tau: From molecular biophysics to physiology and disease.Protein Sci. 2021 Jul;30(7):1294-1314. doi: 10.1002/pro.4093. Epub 2021 May 14. Protein Sci. 2021. PMID: 33930220 Free PMC article. Review.
References
-
- Ginell GM, Holehouse AS. An Introduction to the Stickers-and-Spacers Framework as Applied to Biomolecular Condensates. In: Zhou H-X, Spille J-H, Banerjee PR (eds). Phase-Separated Biomolecular Condensates: Methods and Protocols. Springer; US: New York, NY, 2023, pp 95-116. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
