The Bad Reputation of Digoxin in Atrial Fibrillation-Causality or Bias? Nationwide Nested Case-Control Study
- PMID: 40166486
- PMCID: PMC11957803
- DOI: 10.1016/j.ajmo.2025.100093
The Bad Reputation of Digoxin in Atrial Fibrillation-Causality or Bias? Nationwide Nested Case-Control Study
Abstract
Aims: Studies have reported excess risk of mortality associated with digoxin in atrial fibrillation (AF).This study sought to investigate if these findings could be replicated and whether a potential association could be explained by bias.
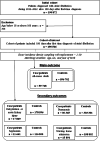
Methods: Using Danish Nationwide registers, a nested-case control study from 2012 to 2022 was conducted in a cohort of patients with AF. Cases were defined as death of any cause and the exposure was treatment with digoxin compared with beta blockers/verapamil. To investigate bias, additional analyses with negative control outcomes as case definitions-in which we would not expect a plausible association (eg, nursing home admission)-were employed. Associations were reported as hazard ratios (HRs) with 95% confidence intervals (95% CI).
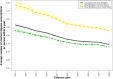
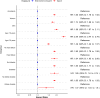
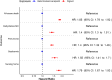
Results: A total of 59,748 cases were identified and matched 1:10 with controls (53% men, median age: 84 [IQR: 77-89]). Digoxin was associated with increased rates of mortality in the entire cohort (HR 1.85, 95% CI 1.78-1.92) as well as subgroups such as patients with heart failure (HR 1.84, 95% CI 1.65-2.06), diabetes (HR 1.85, 95% CI 1.6-2.14), and kidney disease (HR 1.37, 95% CI 1.04-1.8). Significant associations with all negative control outcomes were also found, most notably nursing home admissions (HR 1.79, 95% CI 1.67-1.93).
Conclusion: Digoxin use was associated with increased mortality in AF. However, negative control outcomes were also associated with digoxin use indicating that the described association between digoxin and mortality is likely not causal and being prescribed digoxin is merely a marker of more advanced disease and frailty.
Keywords: Atrial fibrillation; Digoxin; Negative control outcomes; Pharmacoepidemiology; Residual confounding.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Van Gelder I.C., Rienstra M., Bunting K.V., et al. 2024 ESC guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2024;45:3314–3414. - PubMed
-
- Gheorghiade M., Adams K.F., Colucci W.S. Digoxin in the management of cardiovascular disorders. Circulation. 2004;109:2959–2964. - PubMed
-
- Antman E.M., Wenger T.L., Butler V.P., et al. Treatment of 150 cases of life-threatening digitalis intoxication with digoxin-specific Fab antibody fragments. Final report of a multicenter study. Circulation. 1990;81:1744–1752. - PubMed
-
- Bauman J.L., DiDomenico R.J., Galanter W.L. Mechanisms, manifestations, and management of digoxin toxicity in the modern era. Am J Cardiovasc Drugs. 2006;6:77–86. - PubMed
-
- Hallberg P., Lindbäck J., Lindahl B., et al. for the RIKS-HIA group. Digoxin and mortality in atrial fibrillation: a prospective cohort study. Eur J Clin Pharmacol. 2007;63:959–971. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

