PCSK9 Manipulates Lipid Metabolism and the Immune Microenvironment in Cancer
- PMID: 40166624
- PMCID: PMC11956896
- DOI: 10.2147/OTT.S504637
PCSK9 Manipulates Lipid Metabolism and the Immune Microenvironment in Cancer
Abstract
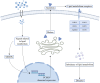
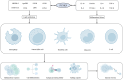
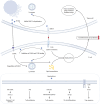
Cancer remains the foremost cause of mortality on a global scale. Immunotherapy has yielded remarkable outcomes in the fight against cancer and is regarded as one of the most crucial and promising therapeutic modalities. PCSK9, a critical target for plasma lipids control, has been extensively and deeply studied in multiple diseases. Currently, the functions of PCSK9 in cancer, particularly its immunomodulatory role, have been progressively revealed. PCSK9 is capable of modulating a variety of immune response throughout tumor progression by orchestrating lipid metabolism. Moreover, PCSK9 governs the cell fate of diverse immune cells, such as inflammatory factor signals, MHC signals, and TCR signals. This review comprehensively summarizes the current state of knowledge regarding the role and underlying mechanisms of PCSK9 in tumorigenesis, progression, immune escape, and drug resistance.
Keywords: PCSK9; cancer; immunotherapy; lipid metabolism.
© 2025 Cui et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures
Similar articles
-
The multifaceted role of PCSK9 in cancer pathogenesis, tumor immunity, and immunotherapy.Med Oncol. 2024 Jul 15;41(8):202. doi: 10.1007/s12032-024-02435-0. Med Oncol. 2024. PMID: 39008137 Review.
-
Targeting PCSK9 to upregulate MHC-II on the surface of tumor cells in tumor immunotherapy.BMC Cancer. 2024 Apr 10;24(1):445. doi: 10.1186/s12885-024-12148-2. BMC Cancer. 2024. PMID: 38600469 Free PMC article.
-
Identification and validation of PCSK9 as a prognostic and immune-related influencing factor in tumorigenesis: a pan-cancer analysis.Front Oncol. 2023 Oct 4;13:1134063. doi: 10.3389/fonc.2023.1134063. eCollection 2023. Front Oncol. 2023. PMID: 37860186 Free PMC article.
-
Integrated bioinformatics analysis identifies PCSK9 as a prognosticator correlated with lipid metabolism in pancreatic adenocarcinoma.World J Surg Oncol. 2024 Sep 28;22(1):256. doi: 10.1186/s12957-024-03532-0. World J Surg Oncol. 2024. PMID: 39342295 Free PMC article.
-
Current Understanding of PCSK9 and Its Relevance to Cancer Prognosis and Immune Therapy: A Review.Iran J Pathol. 2024 Winter;19(1):1-9. doi: 10.30699/IJP.2023.1999459.3093. Epub 2023 Dec 29. Iran J Pathol. 2024. PMID: 38864086 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

