Disproportionality analysis of interstitial lung disease associated with novel antineoplastic agents during breast cancer treatment: a pharmacovigilance study
- PMID: 40166653
- PMCID: PMC11957809
- DOI: 10.1016/j.eclinm.2025.103160
Disproportionality analysis of interstitial lung disease associated with novel antineoplastic agents during breast cancer treatment: a pharmacovigilance study
Abstract
Background: Studies have shown that some antineoplastic agents may be associated with interstitial lung disease (ILD), but large-scale real-world data are lacking. This study aimed to detect signals of disproportionate reporting for ILD associated with novel antineoplastic agents used in breast cancer treatment.
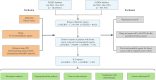
Methods: In this pharmacovigilance study, we collected data from the FDA Adverse Event Reporting System (FAERS; Jan 01, 2004-Dec 31, 2023) and the Japanese Adverse Drug Event Report (JADER; Jan 01, 2004-Mar 31, 2024) databases. Data retrieval involved direct download of structured datasets from the FDA and PMDA portals. Participant selection included reports of FDA-approved novel antineoplastic agents for breast cancer with documented ILD as a preferred term, excluding duplicates, non-breast cancer indications, unapproved drugs, and cases where drugs were classified as concomitant or interacting. Signals of disproportionate reporting were assessed using the reporting odds ratio (ROR), with statistical significance defined as a lower 95% confidence interval >1 and ≥3 ILD cases.
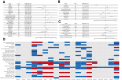
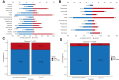
Findings: A total of 2913 patients with ILD from FAERS and 1868 from JADER were analysed. We identified 9 agents with reporting signals for ILD in FAERS: ROR and 95% confidence interval (CI) for trastuzumab deruxtecan was 12.17 (95% CI 11.04-13.41), atezolizumab 6.04 (5.02-7.28), everolimus 3.21 (2.95-3.50), abemaciclib 2.87 (2.52-3.27), pertuzumab 2.84 (2.49-3.25), olaparib 2.29 (1.65-3.19), trastuzumab emtansine 2.27 (1.91-2.69), pembrolizumab 2.06 (1.65-2.58), and trastuzumab 1.36 (1.25-1.49). 7 drugs associated with ILD in JADER are also captured in FAERS. Fatal cases presented with a shorter median onset time compared to nonfatal cases (56 vs. 71 days in FAERS, P = 0.015; 59 vs. 76.5 days in JADER, P = 0.046). Analyses indicated stronger reporting associations between novel antineoplastic agents and ILD compared to chemotherapeutics (FAERS: OR 2.47, 2.16-2.81; JADER: OR 1.61, 1.37-1.88; P < 0.0001). ILD reports were more frequent among older patients (FAERS: HR 1.0097, 1.0036-1.0159, P = 0.0020; JADER: HR 1.0183, 1.0094-1.0270, P < 0.0001), while higher weight correlated with fewer reports (FAERS: HR 0.9783, 0.9729-0.9836; P < 0.0001).
Interpretation: Our study detected signals of disproportionate reporting for ILD with some novel antineoplastic agents in breast cancer, fatal cases had a shorter median onset time than nonfatal ones. Novel antineoplastic agents showed stronger signal of disproportionate reporting associations with ILD than chemotherapeutics. Older age and lower weight were associated with more frequent ILD reports. The limitations-including incomplete data, inherent pharmacovigilance biases, and coprescription bias-preclude causal interpretation of the observed associations and may lead to overestimation or underestimation of reporting signals. These findings highlight the need for vigilant ILD monitoring but require validation through prospective studies to clarify true clinical risks.
Funding: None.
Keywords: Adverse event; Breast cancer; Interstitial lung disease; Novel antineoplastic agent.
© 2025 The Authors.
Conflict of interest statement
All authors have declared no conflicts of interest.
Figures


References
-
- Hurvitz S.A., Andre F., Jiang Z., et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16:816–829. - PubMed
-
- Schmid P., Cortes J., Dent R., et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556–567. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

