Concizumab prophylaxis in people with hemophilia A or B without inhibitors: patient-reported outcome results from the phase 3 explorer8 study
- PMID: 40166710
- PMCID: PMC11957488
- DOI: 10.1016/j.rpth.2025.102705
Concizumab prophylaxis in people with hemophilia A or B without inhibitors: patient-reported outcome results from the phase 3 explorer8 study
Abstract
Background: Patient-reported outcomes (PROs) can provide useful insights into patient perception of concizumab, an anti-tissue factor pathway inhibitor monoclonal antibody intended for once-daily, subcutaneous prophylaxis for hemophilia A (HA) or hemophilia B (HB), with and without inhibitors.
Objectives: To evaluate PROs from the phase 3 explorer8 study (NCT04082429).
Methods: Male patients aged ≥12 years with HA/HB without inhibitors were enrolled and randomized 1:2 to no prophylaxis/on-demand treatment (arm 1) or concizumab prophylaxis (arm 2) or allocated to concizumab prophylaxis (arms 3 and 4). PRO questionnaires included the 36-item short-form health survey version 2, Haemophilia Quality of Life Questionnaire for Adults, Hemophilia Treatment Experience Measure, and Haemophilia Patient Preference Questionnaire.
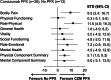
Results: Estimated treatment difference for change in 36-item short-form health survey version 2 "bodily pain" and "physical functioning" from baseline to week 24 between patients in arms 1 and 2 was 9.5 points (95% CI, 2.4 to 16.7) and 0.3 points (95% CI, -5.1 to 5.6), respectively. Estimated treatment difference at week 24 between patients in arms 1 and 2 was -18.0 points (95% CI, -26.4 to -9.5) for Haemophilia Quality of Life Questionnaire for Adults "total score" and -16.8 points (95% CI, -32.2 to -1.4) for "physical health." Hemophilia Treatment Experience Measure and Haemophilia Patient Preference Questionnaire results favored concizumab prophylaxis over no prophylaxis or previous treatment.
Conclusion: PRO data from the phase 3 explorer8 study provided additional support for concizumab prophylaxis compared with no prophylaxis as a treatment option for patients with HA/HB.
Keywords: concizumab; health-related quality of life; hemophilia A; hemophilia B; patient-reported outcomes.
© 2025 The Authors.
Figures




Similar articles
-
Concizumab prophylaxis in persons with hemophilia A or B with inhibitors: patient-reported outcome results from the phase 3 explorer7 study.Res Pract Thromb Haemost. 2024 Jun 17;8(4):102476. doi: 10.1016/j.rpth.2024.102476. eCollection 2024 May. Res Pract Thromb Haemost. 2024. PMID: 39099801 Free PMC article.
-
Concizumab prophylaxis in people with haemophilia A or haemophilia B without inhibitors (explorer8): a prospective, multicentre, open-label, randomised, phase 3a trial.Lancet Haematol. 2024 Dec;11(12):e891-e904. doi: 10.1016/S2352-3026(24)00307-7. Epub 2024 Nov 6. Lancet Haematol. 2024. PMID: 39521008 Clinical Trial.
-
Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results.Blood. 2019 Nov 28;134(22):1973-1982. doi: 10.1182/blood.2019001542. Blood. 2019. PMID: 31444162 Free PMC article. Clinical Trial.
-
Concizumab, a Non-Replacement Therapy for Persons with Hemophilia with Inhibitors.J Clin Med. 2025 Apr 25;14(9):2961. doi: 10.3390/jcm14092961. J Clin Med. 2025. PMID: 40363993 Free PMC article. Review.
-
Evaluating the Safety and Efficacy of Concizumab in Hemophilia A/B Patients: A Systematic Review.Clin Appl Thromb Hemost. 2025 Jan-Dec;31:10760296251342968. doi: 10.1177/10760296251342968. Epub 2025 May 14. Clin Appl Thromb Hemost. 2025. PMID: 40368339 Free PMC article.
References
-
- Gringeri A., von Mackensen S. Quality of life in haemophilia. Haemophilia. 2008;14(Suppl 3):19–25. - PubMed
-
- Wyrwich K.W., Krishnan S., Poon J.L., Auguste P., von Maltzahn R., Yu R., et al. Interpreting important health-related quality of life change using the Haem-A-QoL. Haemophilia. 2015;21:578–584. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

