A Randomized Controlled Trial of Thoracentesis in Acute Heart Failure
- PMID: 40166829
- PMCID: PMC12011436
- DOI: 10.1161/CIRCULATIONAHA.124.073521
A Randomized Controlled Trial of Thoracentesis in Acute Heart Failure
Abstract
Background: TAP-IT (Thoracentesis to Alleviate Cardiac Pleural Effusion-Interventional Trial) investigated the effect of therapeutic thoracentesis in addition to standard medical therapy in patients with acute heart failure and sizeable pleural effusion.
Methods: This multicenter, unblinded, randomized controlled trial, conducted between August 31, 2021, and March 22, 2024, included patients with acute heart failure, left ventricular ejection fraction ≤45%, and non-negligible pleural effusion. Patients with very large effusions (more than two-thirds of the hemithorax) were excluded. Participants were randomly assigned 1:1 to upfront ultrasound-guided pleural pigtail catheter thoracentesis in addition to standard medical therapy or standard medical therapy alone. The primary outcome was days alive out of the hospital over the following 90 days; key secondary outcomes included length of admission and 90-day all-cause mortality. All outcomes were analyzed according to the intention-to-treat principle.
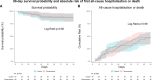
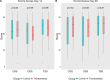
Results: A total of 135 patients (median age, 81 years [25th; 75th percentile, 75; 83]; 33% female; median left ventricular ejection fraction, 25% [25th; 75th percentile, 20%; 35%]) were randomized to either thoracentesis (n=68) or standard medical therapy (n=67). The thoracentesis group had a median of 84 days (77; 86) alive out of the hospital over the following 90 days compared with 82 days (73; 86) in the control group (P=0.42). The mortality rate was 13% in both groups, with no difference in survival probability (P=0.90). There were no differences in the duration of the index admission (control group median, 5 days [3; 8]; thoracentesis group median, 5 days [3; 7], P=0.69). Major complications occurred in 1% of thoracenteses performed during the study period.
Conclusions: For patients with acute heart failure and pleural effusion, a strategy of upfront routine thoracentesis in addition to standard medical therapy did not increase days alive out of the hospital for 90 days, all-cause mortality, or duration of index admission. The current findings lay the groundwork for future research to confirm the results.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT05017753.
Keywords: heart failure; pleural effusion; thoracentesis.
Conflict of interest statement
Dr Bang reports congress travel, speaker, and advisory board funding from Boehringer Ingelheim; speaker and congress travel funding from Novo Nordisk; and congress travel funding from Orion Pharma and Bayer. Dr Schou reports lecture fees from Novo Nordisk, Novartis, AstraZeneca, and Boehringer Ingelheim. Drs Løgstrup and Vraa report speaker funding from Boehringer Ingelheim, Novartis, and AstraZeneca. Dr Stride reports advisory board and congress travel funding from Boehringer Ingelheim and AstraZeneca. Dr Køber reports speaker honoraria from AstraZeneca, Boehringer, Novartis, and Novo Nordisk. Dr Gustafsson reports serving as an advisor for Abbott, Bayer, AstraZeneca, Pfizer, Alnylam, Ionis, Boehringer Ingelheim, AdjuCor, and Corwave, and as a speaker for Novartis, all outside the current work. Dr Thune reports congress travel and honoraria for lectures from AstraZeneca and honoraria for lectures from BMS. Drs Glargaard, Thomsen, Tuxen, Lindholm, Iversen, Rasmussen, Seven, Barasa, Tofterup, Høfsten, and Rossing report no disclosures.
Figures


References
-
- Morales-Rull JL, Bielsa S, Conde-Martel A, Aramburu-Bodas O, Llàcer P, Quesada MA, Suárez-Pedreira I, Manzano L, Montero-Pérez Barquero M, Porcel JM. Pleural effusions in acute decompensated heart failure: prevalence and prognostic implications. Eur J Intern Med. 2018;52:49–53. doi: 10.1016/j.ejim.2018.02.004 - PubMed
-
- Kataoka H. Pericardial and pleural effusions in decompensated chronic heart failure. Am Heart J. 2000;139:918–923. doi: 10.1016/s0002-8703(00)90026-7 - PubMed
-
- Porcel JM, Vives M. Letters to the Editor: Distribution of pleural effusion in congestive heart failure. South Med J. 2006;99:98–99. doi: 10.1097/01.smj.0000199278.81401.2f - PubMed
-
- Glargaard S, Deis T, Abild-Nielsen AG, Stark A, Thomsen JH, Kristensen SL, Rossing K, Gustafsson F, Thune JJ. Pleural effusion and invasive hemodynamic measurements in advanced heart failure. Circ Heart Fail. 2024;17:e011253. doi: 10.1161/CIRCHEARTFAILURE.123.011253 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

