The Antiviral Activity of Polyphenols
- PMID: 40166854
- PMCID: PMC12319496
- DOI: 10.1002/mnfr.70042
The Antiviral Activity of Polyphenols
Abstract
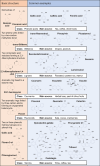
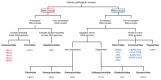
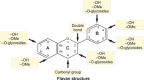
Polyphenols are secondary metabolites produced by a large variety of plants. These compounds that comprise the class of phenolic acids, stilbenes, lignans, coumarins, flavonoids, and tannins have a wide range of employment, from food production to medical usages. Among the beneficial applications of polyphenols, their antiviral activity is gaining importance due to the increased prevalence of drug-resistant viruses such as herpes and hepatitis B viruses. In the present review, we provide an overview of the most promising or commonly used antiviral polyphenols and their mechanisms of action focusing on their effects on enveloped viruses of clinical importance (double-stranded linear or partially double-stranded circular DNA viruses, negative sense single-stranded RNA viruses with nonsegmented or segmented genomes, and positive sense single-stranded RNA viruses). The present work emphasizes the relevance of polyphenols, in particular epigallocatechin-3-gallate and resveratrol, as alternative or supportive antivirals. Polyphenols could interfere with virtually all steps of viral infection, from the adsorption to the release of viral particles. The activity of polyphenols against viruses is especially relevant given the risk of widespread outbreaks associated with viruses, remarked by the recent COVID-19 pandemic.
Keywords: antivirals; enveloped virus; polyphenols.
© 2025 The Author(s). Molecular Nutrition & Food Research published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- El Gharras H., “Polyphenols: food sources, properties and applications – a review,” International Journal of Food Science and Technology 44 (2009): 2512, 10.1111/j.1365-2621.2009.02077.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

