Endothelial MICU1 protects against vascular inflammation and atherosclerosis by inhibiting mitochondrial calcium uptake
- PMID: 40166941
- PMCID: PMC11957702
- DOI: 10.1172/JCI181928
Endothelial MICU1 protects against vascular inflammation and atherosclerosis by inhibiting mitochondrial calcium uptake
Abstract
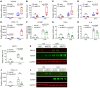
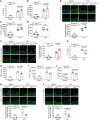
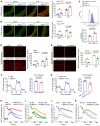
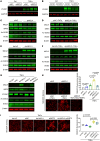
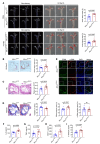
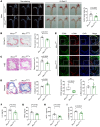
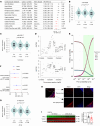
Mitochondrial dysfunction fuels vascular inflammation and atherosclerosis. Mitochondrial calcium uptake 1 (MICU1) maintains mitochondrial Ca2+ homeostasis. However, the role of MICU1 in vascular inflammation and atherosclerosis remains unknown. Here, we report that endothelial MICU1 prevents vascular inflammation and atherosclerosis by maintaining mitochondrial homeostasis. We observed that vascular inflammation was aggravated in endothelial cell-specific Micu1 knockout mice (Micu1ECKO) and attenuated in endothelial cell-specific Micu1 transgenic mice (Micu1ECTg). Furthermore, hypercholesterolemic Micu1ECKO mice also showed accelerated development of atherosclerosis, while Micu1ECTg mice were protected against atherosclerosis. Mechanistically, MICU1 depletion increased mitochondrial Ca2+ influx, thereby decreasing the expression of the mitochondrial deacetylase sirtuin 3 (SIRT3) and the ensuing deacetylation of superoxide dismutase 2 (SOD2), leading to the burst of mitochondrial reactive oxygen species (mROS). Of clinical relevance, we observed decreased MICU1 expression in the endothelial layer covering human atherosclerotic plaques and in human aortic endothelial cells exposed to serum from patients with coronary artery diseases (CAD). Two-sample Wald ratio Mendelian randomization further revealed that increased expression of MICU1 was associated with decreased risk of CAD and coronary artery bypass grafting (CABG). Our findings support MICU1 as an endogenous endothelial resilience factor that protects against vascular inflammation and atherosclerosis by maintaining mitochondrial Ca2+ homeostasis.
Keywords: Atherosclerosis; Cell biology; Vascular biology.
Figures

References
-
- Ridker PM, et al. Inflammation and atherosclerosis. Circulation. 2023;105(9):1135–1143. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

