Genetic Testing Utilization in the U.S. Registry for Childhood Interstitial and Diffuse Lung Diseases
- PMID: 40167520
- PMCID: PMC11960725
- DOI: 10.1002/ppul.71073
Genetic Testing Utilization in the U.S. Registry for Childhood Interstitial and Diffuse Lung Diseases
Abstract
Introduction: Childhood interstitial and diffuse lung diseases (chILD) comprise a diverse group of rare disorders. Identifying the underlying cause is crucial for treatment, prognosis, and estimating recurrence risk. The objective of this study was to assess the utilization of genetic testing for subjects enrolled in the United States National Registry for ChILD, a multicenter observational study.
Methods: Genetic data from participating sites were reviewed and analyzed in relationship to clinical characteristics.
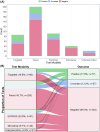
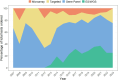
Results: Of 609 children enrolled from 22 centers, genetic testing was performed for 55.5% (n = 338). Genetic testing results were positive (diagnostic) for 22.8% (n = 77), negative for 60.7% (n = 205), and uncertain for 16.6% (n = 56). Most testing was performed through gene panels (55.9%), followed by exome sequencing (ES) or whole genome sequencing (WGS) (26.9%), single gene testing (24.6%), and/or chromosomal microarray (11.8%). For participants with positive (diagnostic) genetic testing results, the majority were diagnosed through gene panel (33.8%; n = 26) or single gene testing (32.5%; n = 25). The most common diagnosis confirmed by genetic testing was SFTPC-associated surfactant metabolism dysfunction. Of the 59 subjects with unclassified ILD, only 22% (n = 13) had undergone ES or WGS, 61% (n = 36) had received panel testing, and 27% (n = 16) did not have any genetic testing reported.
Conclusion: The utilization of genetic testing has been variable in infants and children enrolled in the ChILD Registry. Additional efforts are needed to develop genetic testing recommendations for children with suspected ILD. Furthermore, there is opportunity for broader utilization of ES/WGS and genetic discovery for children with lung disease of unclear etiology.
Keywords: childhood interstitial lung disease (chILD); genetic testing; rare lung diseases.
© 2025 The Author(s). Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
L.R.Y.: receives royalties from Wolters Kluwer for authorship in UpToDate, consultant for Boehringer Ingelheim pediatric advisory board (outside the submitted work). J.B.T.‐W.: site investigator for Astra Zeneca (not related to interstitial lung disease). G.H.D.: Pathology consultant for Boehringer Ingelheim InPedILD trial; Pathology consultant for 4DMT. M.R.P – Site Investigator for Vertex Pharmaceuticals (not related to interstitial lung disease). W.A.G.: receives royalties from Wolters Kluwer for authorship in UpToDate. R.R.D.: receives royalties from Elsevier for editorship of Kendig and Wilmott's Disorders of the Respiratory Tract in Children, consultation for Boehringer Ingelheim pediatric advisory board and principal investigator for the INPEDILD and INPEDILD‐ ON trial. All other authors report no conflicts of interest.
Figures

References
-
- Nathan N., Borensztajn K., and Clement A., “Genetic Causes and Clinical Management of Pediatric Interstitial Lung Diseases,” Current Opinion in Pulmonary Medicine 24, no. 3 (2018): 253–259. - PubMed
-
- Kurland G., Deterding R. R., Hagood J. S., et al., “An Official American Thoracic Society Clinical Practice Guideline: Classification, Evaluation, and Management of Childhood Interstitial Lung Disease in Infancy,” American Journal of Respiratory and Critical Care Medicine 188, no. 3 (2013): 376–394. - PMC - PubMed
-
- Bush A., Cunningham S., de Blic J., et al., “European Protocols for the Diagnosis and Initial Treatment of Interstitial Lung Disease in Children,” Thorax 70, no. 11 (2015): 1078–1084. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

