Patient- and Community-Level Characteristics Associated With Respiratory Syncytial Virus Vaccination
- PMID: 40168024
- PMCID: PMC11962666
- DOI: 10.1001/jamanetworkopen.2025.2841
Patient- and Community-Level Characteristics Associated With Respiratory Syncytial Virus Vaccination
Abstract
Importance: In 2023, the first respiratory syncytial virus (RSV) vaccines were recommended for US adults 60 years or older, but few data are available about which patients were most likely to receive vaccine to inform future RSV vaccine outreach efforts.
Objective: To assess patient- and community-level characteristics associated with RSV vaccine receipt and patient knowledge and attitudes related to RSV disease and RSV vaccines.
Design, setting, and participants: During the first season of RSV vaccine use from October 1, 2023, to April 30, 2024, adults 60 years or older hospitalized with RSV-negative acute respiratory illness were enrolled in this cross-sectional study from 26 hospitals in 20 US states. Sociodemographic and clinical data were abstracted from health records, and structured interviews were conducted for knowledge and attitudes about RSV disease and RSV vaccines.
Exposures: Age, sex, race and ethnicity, pulmonary disease, immunocompromised status, long-term care facility residence, medical insurance, social vulnerability index (SVI), and educational level.
Main outcomes and measures: The exposures were identified a priori as possible factors associated with RSV vaccine receipt and were entered into a modified Poisson regression model accounting for state clustering, to assess for association with RSV vaccine receipt. Knowledge and attitudes were summarized with frequencies and proportions.
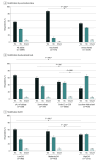
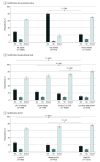
Results: Among 6746 hospitalized adults 60 years or older, median age was 73 (IQR, 66-80) years and 3451 (51.2%) were female. Among the 6599 patients with self-reported race and ethnicity, 699 (10.6%) were Hispanic, 1288 (19.5%) were non-Hispanic Black, 4299 (65.1%) were non-Hispanic White, and 313 (4.7%) were other race or ethnicity. There were 700 RSV-vaccinated (10.4%) and 6046 unvaccinated (89.6%) adults. Among 3219 unvaccinated adults who responded to RSV knowledge questions, 1519 (47.2%) had not heard of RSV or were unsure; 2525 of 3218 (78.5%) were unsure if they were eligible for RSV vaccine or thought they were not. In adjusted analyses, characteristics associated with RSV vaccination were being 75 years or older (adjusted risk ratio [ARR], 1.23; 95% CI, 1.10-1.38, P < .001), being male (ARR, 1.15; 95% CI, 1.01-1.30; P = .04), and having pulmonary disease (ARR, 1.39; 95% CI, 1.16-1.67; P < .001), immunocompromised status (ARR, 1.30; 95% CI, 1.14-1.48; P < .001), low (ARR, 1.47; 95% CI, 1.18-1.83, P < .001) or moderate (ARR, 1.47; 95% CI, 1.21-1.79; P < .001) SVI, and educational level consisting of 4 or more years of college (ARR, 2.91; 95% CI, 2.14-3.96; P < .001), at least some college or technical training (ARR, 1.85; 95% CI, 1.35-2.53; P < .001), or grade 12 education or General Educational Development (ARR, 1.44; 95% CI, 1.03-2.00; P = .03). RSV vaccination was less likely among residents of long-term care facilities, patients with Medicaid coverage, and uninsured patients.
Conclusions and relevance: In this cross-sectional study of hospitalized adults, knowledge of RSV disease and RSV vaccine eligibility was low. Older adults and those with certain medical conditions were more likely to have received vaccine, suggesting appropriate prioritization, but sociodemographic differences in vaccine uptake occurred.
Conflict of interest statement
Figures
References
-
- Surie D, Yuengling KA, DeCuir J, et al. ; Investigating Respiratory Viruses in the Acutely Ill (IVY) Network . Severity of respiratory syncytial virus vs COVID-19 and influenza among hospitalized US adults. JAMA Netw Open. 2024;7(4):e244954. doi: 10.1001/jamanetworkopen.2024.4954 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

