Fidaxomicin for Clostridioides difficile infection in patients with inflammatory bowel disease: a multicenter retrospective cohort study
- PMID: 40168072
- PMCID: PMC12060865
- DOI: 10.1093/ecco-jcc/jjaf056
Fidaxomicin for Clostridioides difficile infection in patients with inflammatory bowel disease: a multicenter retrospective cohort study
Erratum in
-
Correction to: Fidaxomicin for Clostridioides difficile infection in patients with inflammatory bowel disease: a multicenter retrospective cohort study.J Crohns Colitis. 2025 Sep 7;19(8):jjaf123. doi: 10.1093/ecco-jcc/jjaf123. J Crohns Colitis. 2025. PMID: 40971653 Free PMC article. No abstract available.
Abstract
Background and aims: Inflammatory bowel disease (IBD) patients with Clostridioides difficile infection (CDI) are at increased risk of adverse outcomes. Data on fidaxomicin use in IBD remain scarce. We assessed the effectiveness and safety of fidaxomicin for CDI and its impact on IBD outcomes in a large international cohort.
Methods: Adult patients with ulcerative colitis (UC) or Crohn's disease (CD) treated with fidaxomicin for documented CDI were retrospectively included. The primary outcome was CDI recurrence rate within 8 weeks (C. difficile toxin detection and CDI-targeted therapy). Secondary outcomes included sustained response (no CDI-targeted therapy within 12 weeks), IBD therapy escalation, colectomy rate, and all-cause mortality within 30, 90, and 180 days.
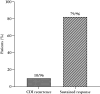
Results: Ninety-six patients (57 UC and 39 CD) from 20 IBD centers were included. Most were on advanced IBD therapy. Half had a previous CDI episode, 15% a severe episode. CDI recurrence rate was 10% at week 8, and sustained response 82% at week 12. Compared with patients with previous CDI episode, patients at first episode tended to have a lower recurrence (4.3% vs 16%; P = .06) and higher sustained response (91% vs 75%; P = .04) rate. IBD therapy escalation was required in 48% with a numerically lower need for patients achieving vs not-achieving sustained response within 30 days (12% vs 20%; P = .42). Five UC patients underwent colectomy. One death unrelated to CDI or IBD occurred. One moderate and 5 mild adverse events were reported.
Conclusions: Fidaxomicin was effective and safe in IBD patients with CDI, with greater effectiveness in CDI-naïve patients, potentially influencing short-term IBD outcomes.
Keywords: Clostridioides difficile; CDI; fidaxomicin; inflammatory bowel disease.
© The Author(s) 2025. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
D.N. received unrestricted research grants from Pfizer, consulting fees from Dr. Falk and Abbvie, and lecturer fees from Ferring and Alfasigma.
M.C. has served as speaker, consultant, or research or education funding from MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Biogen, Gilead, and Lilly.
C.V. received consultancy and lecture fees from: AbbVie, Galapagos, Janssen-Cilag, Johnson & Johnson, Pfizer, Takeda, Celltrion, Alfasigma, Eli Lilly, and research grant from Celltrion and Pfizer.
S.S. has received as lecture fees from and served on the advisory board and as a consultant for AbbVie, Arena, Ferring, Gilead, Janssen, MSD, and Takeda.
A.B. has served as speaker and consultant for Abbvie, Aengus, Boston Scientific, Bristol-Myers Squibb, Dr. Falk Pharma, Genericon, Gilead, Janssen, Lilly, MSD, Olympus, Pfizer, PSI, Sandoz, Takeda, and Vifor.
J.P.G. has served as speaker, consultant, and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers, Gilead/Galapagos, Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine, and Vifor Pharma.
B.B. has served as speaker for AbbVie, Agave, Alfasigma, AG Pharma, Janssen, MSD, Pfizer, Sofar, Takeda, and Unifarco and as a consultant for AbbVie and Janssen.
H.Y. Advisory Committee or Review Panels: Takeda, Abbvie, Pfizer, Janssen, BMS, and Eli Lilli; Grant/Research Support: Pfizer; Speaking and Teaching: Abbvie, Pfizer, Takeda, Novartis, and BMS.
R.F.I. has served as speaker, consultant, or research or education funding from MSD, Abbvie, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Casen recordati, and Otsuka Pharmaceutical.
A.O. Consulting/advisory board fees from AbbVie, Alfa-Sigma, Biogen, Eli-Lilly, Ferring, Galapagos, Giuliani, Janssen, Johnson & Johnson, Lionhealth, MSD, Nestlé, Pfizer, Samsung Bioepis, Sandoz, and Takeda. Speaker’s fees from AbbVie, Alfa-Sigma, Biogen, Celltrion, Eli-Lilly, Ferring, Fresenius Kabi, Galapagos, Janssen, Lionealth, MSD, Pfizer, Samsung Bioepis, Sandoz, and Takeda. Research grants from Takeda and Pfizer.
C.B. served as a consultant or received lecture fees from AbbVie, Alfasigma, Celltrion, Eli Lilly, Ferring, Fresenius Kabi, Galapagos, Lionhealth, Johnson & Johnson, MSD, Pfizer, and Takeda.
A.Z. received lecture fees from AbbVie, Galapagos, Janssen, Tillots Pharma, and Takeda and served as a consultant for AbbVie and Galapagos.
M.a.V. has served as speaker for Abbvie, AGPharma, Alfasigma, Biogen, Celltrion, Galapagos, Johnson&Johnson, Eli Lilly, Ferring, Malesci, Pfizer, Sofar, Takeda; has served as consultant for Abbvie, Biogen, Bristol-Myers Squibb, Celltrion, Eli Lilly, Giuliani, Johnson&Johnson, Pfizer, Sofar, and Takeda; received research support from Giuliani, Pfizer, Sofar, and Takeda.
G.D. reports speaker fees from Alfasigma, Ferring, Johnson& Johnson, Novartis, Pfizer, and Takeda, and has served on the advisory board for AbbVie, Celltrion Healthcare, Eli Lilly, Johnson& Johnson, and Pfizer.
P.B. financial support for research: AbbVie, EG; lecture fees: AbbVie, AMC ICP, Amgen, Bristol Myers Squibb, Celltrion, Dr. Falk Benelux, EG, Galapagos, Globalport, Lilly, Medtalks, Materia Prima, Pentax, Springer Media; advisory board: AbbVie, Bristol Meyers Squibb, CIRC, Galapagos, Janssen, Lilly, Pentax, PSI-CRO, Roche, Takeda, and Tetrameros.
S.V. has received speaker’s fees from AbbVie, Alfasigma, Celltrion, Ferring, Janssen, Lilly, and Takeda.
M.B.A. has served as a speaker, consultant, and advisory member for or has received research funding from MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Alphasigma, Pfizer, Sandoz, Biocon, BMS, Fresenius, Ferring, Tillots, Chiesi, Adacyte, and Oncostellae.
R.A. has served as a speaker, consultant, or received research grants from AbbVie, Abivax, AlfaSigma, AstraZeneca, Bristol-Myers Squibb, Celltrion Healthcare, Dr. Falk Pharma, Galapagos, Johnson & Johnson, Lilly, MSD, Pfizer, and Takeda Pharma.
T.I. received speaker’s fees from Ferring.
E.V.S. has served as speaker for Abbvie, Abivax, Agave, AGPharma, Alfasigma, Apoteca, Biosline, CaDiGroup, Celltrion, Dr. Falk, EG Stada Group, Fenix Pharma, Galapagos, Johnson&Johnson, JB Pharmaceuticals, Innovamedica/Adacyte, Eli Lilly, Malesci, Mayoly Biohealth, Montefarco, Novartis, Omega Pharma, Pfizer, Rafa, Reckitt Benckiser, Sandoz, Sanofi/Regeneron, SILA, Sofar, Takeda, Tillots, Unifarco; has served as consultant for Abbvie, Agave, Alfasigma, Biogen, Bristol-Myers Squibb, Celltrion, Dr. Falk, Eli Lilly, Fenix Pharma, Ferring, Giuliani, Grunenthal, Johnson&Johnson, JB Pharmaceuticals, Merck & Co, Nestlè, Pfizer, Reckitt Benckiser, Sanofi/Regeneron, SILA, Sofar, Takeda, Unifarco; received research support from Bonollo, Difass, Pfizer, Reckitt Benckiser, Sanofi/Regeneron, SILA, Sofar, Unifarco, and Zeta Farmaceutici.
F.C. served as consultant to Abbvie, MSD, Takeda, Janssen, Roche, Celgene, Bristol-Meyers Squibb, Galapagos, Gllead, Pfizer, Mundipharma, Galapagos, and Biogen, received lecture fees from Abbvie, Ferring, Takeda, Allergy Therapeutics, Janssen, Pfizer, and Biogen, and unrestricted research grants from Pfizer, Giuliani, Sofar, MSD, Takeda, and Abbvie.
Figures
References
-
- Singh H, Nugent Z, Yu BN, Lix LM, Targownik LE, Bernstein CN. Higher incidence of clostridium difficile infection among individuals with inflammatory bowel disease. Gastroenterology. 2017;153:430.-. - PubMed
-
- Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443.-. - PubMed
-
- Balram B, Battat R, Al-Khoury A, et al. Risk factors associated with clostridium difficile infection in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2019;13:27.-. - PubMed
-
- Jodorkovsky D, Young Y, Abreu MT. Clinical outcomes of patients with ulcerative colitis and co-existing Clostridium difficile infection. Dig Dis Sci. 2010;55:415.-. - PubMed
-
- Murthy SK, Steinhart AH, Tinmouth J, Austin PC, Daneman N, Nguyen GC. Impact of Clostridium difficile colitis on 5-year health outcomes in patients with ulcerative colitis. Aliment Pharmacol Ther. 2012;36:1032.-. - PubMed