Deep Corneal Nerve Plexus Selective Damage in Persistent Neurotrophic Corneal Epithelial Defects Detected by In Vivo Multiphoton Confocal Microscopy
- PMID: 40168155
- PMCID: PMC11967994
- DOI: 10.1167/iovs.66.4.1
Deep Corneal Nerve Plexus Selective Damage in Persistent Neurotrophic Corneal Epithelial Defects Detected by In Vivo Multiphoton Confocal Microscopy
Abstract
Purpose: To investigate the corneal nerve damage in neurotrophic corneal persistent epithelial defects by an in vivo imaging system using in vivo multiphoton confocal microscopy (MCM) and calcitonin gene-related peptide (CGRP):GFP Tg mice.
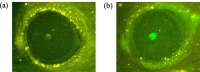
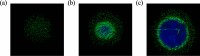
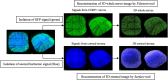
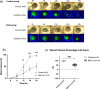
Methods: Corneal epithelium was scraped, followed by administering a single dose of benzalkonium chloride (BAK) to develop a neurotrophic persistent epithelial defect. The defect was imaged with fluorescein staining for up to 24 hours, and wound closure percentage (%, WCP) was calculated. CGRP:GFP Tg mice were used in combination with in vivo MCM to acquire in vivo images of corneal nerve before and 24 hours after the creation of a corneal epithelial defect. GFP signals from CGRP-positive nerves were reconstructed into three-dimensional (3D) images, and nerve volume was analyzed. Additionally, corneal mechanosensation was evaluated using Cochet-Bonnet esthesiometry.
Results: BAK-treated eyes showed a significant delay in WCP at 24 hours. In CGRP:GFP Tg mice, CGRP-positive nerves were successfully captured by in vivo MCM and reconstructed into 3D images. BAK-treated eyes showed a significant decrease in both stromal nerve volume and corneal mechanosensation compared to no BAK eyes at 24 hours after corneal scraping, suggesting that BAK impaired the stromal nerves in both structural and functional asides.
Conclusions: Our in vivo corneal nerve imaging system using the combination of in vivo MCM and CGRP:GFP Tg mice demonstrated a longitudinal observation of murine corneal nerves. This system revealed that corneal stromal nerves were selectively damaged in persistent neurotrophic corneal epithelial defects and offered outstanding potential for various applications.
Conflict of interest statement
Disclosure:
Figures



References
-
- Ruiz-Lozano RE, Hernandez-Camarena JC, Loya-Garcia D, Merayo-Lloves J, Rodriguez-Garcia A.. The molecular basis of neurotrophic keratopathy: diagnostic and therapeutic implications. A review. Ocular Surface. 2021; 19: 224–240. - PubMed
-
- Suzuki K, Saito J, Yanai R, et al. .. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003; 22: 113–133. - PubMed
-
- Dua HS, Said DG, Messmer EM, et al. .. Neurotrophic keratopathy. Prog Retin Eye Res. 2018; 66: 107–131. - PubMed
-
- Malhotra R, Elalfy MS, Kannan R, Nduka C, Hamada S.. Update on corneal neurotisation. Br J Ophthalmol. 2019; 103: 26–35. - PubMed
-
- Soni NG, Jeng BH.. Blood-derived topical therapy for ocular surface diseases. Br J Ophthalmol. 2016; 100: 22–27. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

