Mesenchymal stem cell-specific Sirt1 overexpression prevents sarcopenia induced by 1,25-dihydroxyvitamin D deficiency
- PMID: 40168539
- PMCID: PMC12074815
- DOI: 10.18632/aging.206232
Mesenchymal stem cell-specific Sirt1 overexpression prevents sarcopenia induced by 1,25-dihydroxyvitamin D deficiency
Abstract
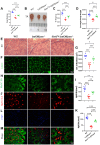
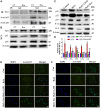
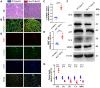
Sarcopenia, characterized by an age-related decline in skeletal muscle mass and function, is closely linked to vitamin D deficiency. This study examines the role of Sirtuin 1 (Sirt1) and its regulation by vitamin D in preventing sarcopenia. Utilizing wild-type, 1α-hydroxylase knockout (1α(OH)ase-/-), and Sirt1 transgenic (Sirt1Tg) 1α(OH)ase-/- mice, we investigated muscle Sirt1 levels, muscle mass, fiber type, and senescence markers. Our results demonstrated that 1,25-Dihydroxyvitamin D (1,25(OH)2D3) upregulated Sirt1 and myogenic factor MyoD1 expression in C2C12 myoblasts via VDR-mediated transcription. Sirt1 overexpression in mesenchymal stem cells (MSCs) significantly mitigated muscle mass reduction, improved fiber cross-sectional area, and increased type II fiber numbers in 1α(OH)ase-/- mice. Mechanistically, 1,25(OH)2D3 promoted muscle cell health by enhancing Sirt1 expression, which in turn reduced muscle cell senescence and the senescence-associated secretory phenotype (SASP) through decreased levels of acetylated nuclear p53 and p65, maintaining their cytoplasmic localization. Additionally, Sirt1 overexpression accelerated muscle regeneration post-injury by increasing embryonic myosin heavy chain expression and cell proliferation. These findings underscore the therapeutic potential of targeting vitamin D and Sirt1 pathways to prevent sarcopenia, suggesting that supplementation with active vitamin D and consequent Sirt1 activation could be effective strategies for managing age-related muscle wasting.
Keywords: Myod1; Sirt1; active vitamin D; muscle regeneration; sarcopenia.
Conflict of interest statement
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

