Adapting Atomic Configuration Steers Dynamic Half-Occupied State for Efficient CO2 Electroreduction to CO
- PMID: 40168680
- PMCID: PMC12006994
- DOI: 10.1021/jacs.5c03121
Adapting Atomic Configuration Steers Dynamic Half-Occupied State for Efficient CO2 Electroreduction to CO
Abstract
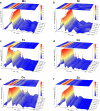
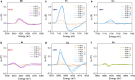
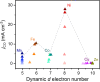
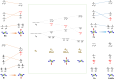
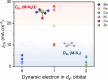
Electronic structures stand at the center to essentially understand the catalytic performance and reaction mechanism of atomically dispersed transition-metal-nitrogen-carbon catalysts (ADTCs). However, under realistic electrocatalytic conditions, the dynamic electronic disturbance at metal centers originating from complicated interactions with microenvironments is commonly neglected, which makes a true structure-property correlation highly ambiguous. Here, we employ operando time-resolved X-ray absorption spectroscopy to delve deeply into dynamic electronic behaviors of a family of transition-metal centers that are observed to adaptively vary in the metal-ligand configuration during the CO2 electroreduction reaction. We identify dynamic electronic/geometric configuration and d-orbital occupation under working conditions, demonstrating an unprecedentedly precise activity descriptor, i.e., dynamic axial dz2 electron, for the CO2-to-CO conversion. Direct results validate that the half-occupied state suggests the optimum binding behaviors with intermediates, significantly promoting CO production, which has been demonstrated by a significant kinetics enhancement of 1 to 2 orders of magnitude as compared with fully occupied and unoccupied states. This work presents the first empirical demonstration for a real correlation between the dynamic electronic/geometric configuration and catalytic kinetics in ADTCs, paving a new way for modulating catalysts and designing highly efficient reaction pathways.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Yang H. B.; Hung S.-F.; Liu S.; Yuan K.; Miao S.; Zhang L.; Huang X.; Wang H.-Y.; Cai W.; Chen R.; Gao J.; Yang X.; Chen W.; Huang Y.; Chen H. M.; Li C. M.; Zhang T.; Liu B. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3 (2), 140–147. 10.1038/s41560-017-0078-8. - DOI
-
- Ju W.; Bagger A.; Hao G.-P.; Varela A. S.; Sinev I.; Bon V.; Roldan Cuenya B.; Kaskel S.; Rossmeisl J.; Strasser P. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 2017, 8 (1), 944. 10.1038/s41467-017-01035-z. - DOI - PMC - PubMed
-
- Jiang Y.; Huang L.; Chen C.; Zheng Y.; Qiao S.-Z. Catalyst–electrolyte interface engineering propels progress in acidic CO2 electroreduction. Energy Environ. Sci. 2025, 18 (5), 2025–2049. 10.1039/D4EE05715E. - DOI
-
- Jiang Y.; Li H.; Chen C.; Zheng Y.; Qiao S.-Z. Dynamic Cu0/Cu+ Interface Promotes Acidic CO2 Electroreduction. ACS Catal. 2024, 14 (11), 8310–8316. 10.1021/acscatal.4c01516. - DOI
LinkOut - more resources
Full Text Sources

