The RBR E3 ubiquitin ligase HOIL-1 can ubiquitinate diverse non-protein substrates in vitro
- PMID: 40169258
- PMCID: PMC11962058
- DOI: 10.26508/lsa.202503243
The RBR E3 ubiquitin ligase HOIL-1 can ubiquitinate diverse non-protein substrates in vitro
Abstract
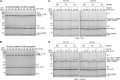
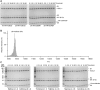
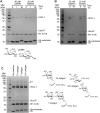
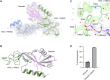
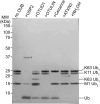
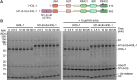
HOIL-1 is a RING-between-RING-family E3 ubiquitin ligase and a component of the linear ubiquitin chain assembly complex. Although most E3 ubiquitin ligases conjugate ubiquitin to protein lysine sidechains, HOIL-1 has also been reported to ubiquitinate hydroxyl groups in protein serine and threonine sidechains and glucosaccharides, such as glycogen and its building block maltose, in vitro. However, HOIL-1 substrate specificity is currently poorly defined. Here, we show that HOIL-1 is unable to ubiquitinate lysine but can efficiently ubiquitinate serine and a variety of model and physiologically relevant di- and monosaccharides in vitro. We identify a critical catalytic histidine residue, His510, in the flexible catalytic site of HOIL-1 that enables this O-linked ubiquitination and prohibits ubiquitin discharge onto lysine sidechains. We use HOIL-1's in vitro non-proteinaceous ubiquitination activity to produce preparative amounts of different ubiquitinated saccharides that can be used as tool compounds and standards in the rapidly emerging field of non-proteinaceous ubiquitination. Finally, we report an engineered, constitutively active HOIL-1 variant that simplifies in vitro generation of ubiquitinated saccharides.
© 2025 Wang et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Akimov V, Barrio-Hernandez I, Hansen SVF, Hallenborg P, Pedersen AK, Bekker-Jensen DB, Puglia M, Christensen SDK, Vanselow JT, Nielsen MM, et al. (2018) UbiSite approach for comprehensive mapping of lysine and N-terminal ubiquitination sites. Nat Struct Mol Biol 25: 631–640. 10.1038/s41594-018-0084-y - DOI - PubMed
-
- Aragão D, Aishima J, Cherukuvada H, Clarken R, Clift M, Cowieson NP, Ericsson DJ, Gee CL, Macedo S, Mudie N, et al. (2018) MX2: A high-flux undulator microfocus beamline serving both the chemical and macromolecular crystallography communities at the Australian Synchrotron. J Synchrotron Radiat 25: 885–891. 10.1107/S1600577518003120 - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
