Total Synthesis and Pharmacological Evaluation of Phochrodines A-C
- PMID: 40169259
- PMCID: PMC12038836
- DOI: 10.1021/acs.jnatprod.5c00104
Total Synthesis and Pharmacological Evaluation of Phochrodines A-C
Abstract
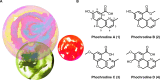
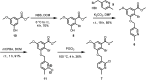
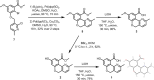
The first syntheses of the Phomopsis-isolated natural products phochrodines A-C are reported. Functional group manipulations on a key 5H-chromeno[4,3-b]pyridine intermediate, itself synthesized from intramolecular Suzuki-Miyaura coupling, enabled facile and high-yielding syntheses of all three natural products. Additionally, sufficient material was generated to enable detailed pharmacological profiling of each compound. Preliminary drug metabolism and pharmacokinetic (DMPK) experiments and ancillary pharmacology screening revealed phochrodine C (3) as an attractive scaffold for further modification, particularly for medicinal chemists working in the antidepressant space.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Meanwell N. A. Improving Drug Candidates by Design: A Focus on Physicochemical Properties as a Means of Improving Compound Disposition and Safety. Chem. Res. Toxicol. 2011, 24, 1420–1456. 10.1021/tx200211v. - DOI - PubMed
- Hann M. M. Molecular Obesity, Potency and Other Addictions in Drug Discovery. Med. Chem. Commun. 2011, 2, 349–355. 10.1039/C1MD00017A. - DOI
-
- Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Delivery Rev. 1997, 23, 3–25. 10.1016/S0169-409X(96)00423-1. - DOI - PubMed
- Veber D. F.; Johnson S. R.; Cheng H.-Y.; Smith B. R.; Ward K. W.; Kopple K. D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. 10.1021/jm020017n. - DOI - PubMed
- Egan W. J.; Merz K. M.; Baldwin J. J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. 10.1021/jm000292e. - DOI - PubMed
- Ghose A. K.; Viswanadhan V. N.; Wendoloski J. J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. 10.1021/cc9800071. - DOI - PubMed
-
-
For selected reviews on this topic, see:
- Sadri A. Is Target-Based Drug Discovery Efficient? Discovery and “Off-Target” Mechanisms of All Drugs. J. Med. Chem. 2023, 66, 12651–12677. 10.1021/acs.jmedchem.2c01737. - DOI - PubMed
- Moffat J. G.; Vincent F.; Lee J. A.; Eder J.; Prunotto M. Opportunities and Challenges in Phenotypic Drug Discovery: An Industry Perspective. Nat. Rev. Drug. Discovery 2017, 16, 531–543. 10.1038/nrd.2017.111. - DOI - PubMed
- Davis R. L. Mechanism of Action and Target Identification: A Matter of Timing in Drug Discovery. iScience 2020, 23, 101487 10.1016/j.isci.2020.101487. - DOI - PMC - PubMed
-
-
-
For selected examples, see:
- Zheng M.; Chen P.; Wu W.; Jiang H. Palladium-Catalyzed Heck-Type Reactions of Oximes with Allylic Alcohols: Synthesis of Pyridines and Azafluorenones. Chem. Commun. 2016, 52, 84–87. 10.1039/C5CC06958K. - DOI - PubMed
- Zhan J.-L.; Wu M.-W.; Wei D.; Wei B.-Y.; Jiang Y.; Yu W.; Han B. 4-HO-TEMPO-Catlayzed Redox Annulation of Cyclopropanols with Oxime Acetates toward Pyridine Derivatives. ACS Catalysis. 2019, 9, 4179–4188. 10.1021/acscatal.9b00832. - DOI
- Stolle W. A. W.; Frissen A. E.; Marcelis A. T. M.; Van der Plas H. C. Intramolecular Diels-Alder Reactions of Pyrimidines and a Computational Study Toward Their Structure and Reactivity. J. Org. Chem. 1992, 57, 3000–3007. 10.1021/jo00037a011. - DOI
- Garrison A. T.; Childress E. S.; Davis D. C.; Lindsley C. W. Preparation of 1,5-Dihydropyrazolo[3′,4′:5,6]pyrano[3,4-b]pyridines via a Microwave-Assisted, Palladium-Catalyzed Regioselective C–H Heteroarylation of Electron-Rich Pyrazoles. J. Org. Chem. 2019, 84, 5855–5862. 10.1021/acs.joc.9b00144. - DOI - PubMed
-
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

