Fast and Autonomous Mannosylated Nanomotors for Dynamic Cancer Cell Targeting
- PMID: 40169377
- PMCID: PMC12124440
- DOI: 10.1002/anie.202505717
Fast and Autonomous Mannosylated Nanomotors for Dynamic Cancer Cell Targeting
Abstract
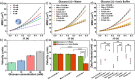
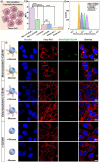
An attractive strategy in cancer cell therapy is to employ motile nanoparticles that can actively search for their target. Herein, we introduce mannosylated compartmentalized cross-linked enzyme-driven nanomotors (c-CLEnM), which exhibit specific and efficient targeting of Hep G2 cells through elevated autonomous motion. In this design, we constructed biodegradable bowl-shaped stomatocytes encapsulating the enzymes glucose oxidase (GOx) and catalase (CAT) within their nanocavity. A subsequent enzyme crosslinking reaction was performed to guarantee their stability. Furthermore, the c-CLEnM were surface modified with a mannose-functional glycopolymer, enabling binding with receptors expressed on Hep G2 cells. Interestingly, the targeting ligands on the nanomotors not only improved their specificity toward cancer cells but also enhanced motility. Compared to the non-mannosylated nanomotors, mannosylated c-CLEnM exhibited enhanced motion and higher targeting efficiency to cells in glucose-containing ionic environments. The unexpected acceleration in speed resulted from the surface modification of these nanomotors with a glycopolymer layer, which increased the zeta potential and created a shielding effect that mitigated the influence of the surrounding ions. This nanomotor design highlights the synergistic effect of functional glycopolymer modification on cellular uptake, adding an additional level of control to nanomotors for application in cancer therapy.
Keywords: Cancer cell targeting; Enhanced autonomous motion; Mannosylated enzymatic nanomotors; Stomatocytes.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dreyfus R., Baudry J., Roper M. L., Fermigier M., Stone H. A., Bibette J., Nature 2005, 437, 862–865. - PubMed
-
- Palagi S., Fischer P., Nat. Rev. Mater. 2018, 3, 113–124.
-
- Sengupta S., Ibele M. E., Sen A., Angew. Chem. Int. Ed. 2012, 51, 8434–8445. - PubMed
-
- Wang W., J. Am. Chem. Soc. 2023, 145, 27185–27197. - PubMed
-
- Ye Z., Wang Y., Liu S., Xu D., Wang W., Ma X., J. Am. Chem. Soc. 2021, 143, 15063–15072. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

