PLA2G7 promotes immune evasion of bladder cancer through the JAK-STAT-PDL1 axis
- PMID: 40169540
- PMCID: PMC11962123
- DOI: 10.1038/s41419-025-07593-1
PLA2G7 promotes immune evasion of bladder cancer through the JAK-STAT-PDL1 axis
Abstract
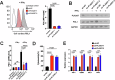
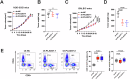
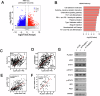
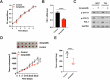
Targeting immune checkpoints such as Programmed death ligand-1 (PD-L1) and Programmed cell death 1 (PD-1) has been approved for treating bladder cancer and shows promising clinical benefits. However, the relatively low response rate highlights the need to seek an alternative strategy to traditional PD-1/PD-L1 targeting immunotherapy. In this study, we found that PLA2G7 is significantly elevated in bladder cancer and correlates with worse prognosis. In vitro experiments demonstrated that knockdown of PLA2G7 does not significantly affect the proliferation, migration, and invasion of bladder cancer cells. Flow cytometry detection, as well as protein and RNA detection, showed that knockdown of PLA2G7 significantly inhibits PD-L1 expression and suppresses the growth of transplanted tumors by promoting CD8 + T-cell infiltration. Further experiments showed that PLA2G7 regulates the JAK-STAT pathway to promote PD-L1 expression by upregulating the phosphorylation of STAT1 and STAT3. Meanwhile, results from syngeneic mouse models indicated that PLA2G7 suppression and anti-CTLA4 therapy have synergistic effects on tumor burden and mouse survival. In addition, we found that ETS1 promotes PLA2G7 overexpression in bladder cancer cells. In summary, our findings provide a novel immunotherapeutic strategy against bladder cancer through targeting the ETS1-PLA2G7-STAT1/STAT3-PD-L1 axis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study was approved by the Biomedical Research Ethics Committee of The First Affiliated Hospital School of Medicine, Zhejiang University (Number: IIT20230241A). All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all participants.
Figures


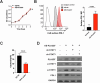
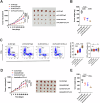
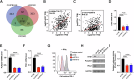
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clinicians. 2023;73:17–48. - PubMed
-
- Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). European Urol. 2022;81:75–94. - PubMed
-
- Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. European Urol. 2021;79:82–104. - PubMed
-
- Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:244–58. - PubMed
MeSH terms
Substances
Grants and funding
- 82303143/National Natural Science Foundation of China (National Science Foundation of China)
- 82403849/National Natural Science Foundation of China (National Science Foundation of China)
- 82172597/National Natural Science Foundation of China (National Science Foundation of China)
- LQ23H160027/Natural Science Foundation of Zhejiang Province (Zhejiang Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

