Mechanical sensing protein PIEZO1 controls osteoarthritis via glycolysis mediated mesenchymal stem cells-Th17 cells crosstalk
- PMID: 40169556
- PMCID: PMC11961634
- DOI: 10.1038/s41419-025-07577-1
Mechanical sensing protein PIEZO1 controls osteoarthritis via glycolysis mediated mesenchymal stem cells-Th17 cells crosstalk
Abstract
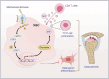
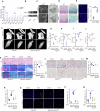
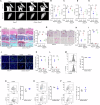
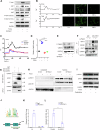
Aberrant mechanical stimuli can cause tissue attrition and activate mechanosensitive intracellular signaling, impacting the progression of osteoarthritis (OA). However, the precise relationship between mechanical loading and bone metabolism remains unclear. Here, we present evidence that Piezo1 senses the mechanical stimuli to coordinate the crosstalk between mesenchymal stem cells (MSCs) and T helper 17 (Th17) cells, leading to the deterioration of bone and cartilage in osteoarthritis (OA). Mechanical loading impaired the property of MSCs by inhibiting their osteo-chondrogenic differentiation and promoting inflammatory signaling to enhance Th17 cells. Mechanistically, mechanical stimuli activated Piezo1, thereby facilitating Ca2+ influx which upregulated the activity of Hexokinase 2(HK2), the rate-limiting enzyme of glycolysis. The resultant increase in glycolytic activity enhanced communication between MSCs and T cells, thus promoting Th17 cell polarization in a macrophage migration inhibitory factor (MIF) dependent manner. Functionally, Wnt1cre; Piezo1fl/fl mice reduced bone and cartilage erosion in the temporomandibular joint condyle following mechanical loading compared to control groups. Additionally, we observed activated Piezo1 and HK2-mediated glycolysis in patients with temporomandibular joint OA, thereby confirming the clinical relevance of our findings. Overall, our results provide insights into how Piezo1 in MSCs coordinates with mechano-inflammatory signaling to regulate bone metabolism. The schema shows that mechanical sensing protein PIEZO1 in MSCs controls osteoarthritis via glycolysis mediated MSCs and Th17 cells crosstalk in a MIF dependent manner.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: The study was approved by the Animal Use and Care Committee of Peking University approval (LA2021488). All institutional and national guidelines for the care and use of laboratory animals were followed. Patient sample collection was approved by the ethics committee of Hospital & School of Stomatology, Peking University (2022-12-83-07).
Figures



References
-
- Vico L, Collet P, Guignandon A, Lafage-Proust M-H, Thomas T, Rehailia M, et al. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–11. - PubMed
-
- Hosseini SM, Wilson W, Ito K, van Donkelaar CC. A numerical model to study mechanically induced initiation and progression of damage in articular cartilage. Osteoarthr Cartil. 2014;22:95–103. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

