Mineralogical controls on PFAS and anthropogenic anions in subsurface soils and aquifers
- PMID: 40169557
- PMCID: PMC11962083
- DOI: 10.1038/s41467-025-58040-w
Mineralogical controls on PFAS and anthropogenic anions in subsurface soils and aquifers
Erratum in
-
Publisher Correction: Mineralogical controls on PFAS and anthropogenic anions in subsurface soils and aquifers.Nat Commun. 2025 Apr 10;16(1):3394. doi: 10.1038/s41467-025-58754-x. Nat Commun. 2025. PMID: 40210630 Free PMC article. No abstract available.
Abstract
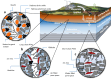
Per- and polyfluoroalkyl substances (PFAS) migrate into the environment through various means, e.g., soil-amendment impurities and ambient atmospheric deposition, potentially resulting in vegetative uptake and migration to groundwater. Existing approaches for modeling sorption of PFAS commonly treat soil as an undifferentiated homogeneous medium, with distribution constants (e.g., Kd, Koc) generated empirically using surface soils. Considering the limited mineral variety expected in weathered geologic media, PFAS mobility can be better understood by accounting for predictable mineral assemblages that are ubiquitously distributed in US soils. Here we explore the role of minerals and electrostatic sorption in controlling PFAS mobility in subsurface settings at contaminated agricultural sites by measuring geochemical parameters and PFAS, and calculating pH-dependent mineral surface charges through full soil and aquifer columns. These data suggest subsurface mobility of short-chain PFAS largely is controlled by aluminum-oxide mineral(oid) electrostatic sorption, whereas long-chain PFAS mobility is controlled by organic matter and air-water interfacial area.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Lu, Q., He, Z. L. & Stoffella, P. J. Land application of biosolids in the USA: a review. Appl. Environ. Soil Sci.2012, 10.1155/2012/201462 (2012).
-
- Clarke, B. O. & Smith, S. R. Review of ‘emerging’ organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ. Int.37, 226–247 (2011). - PubMed
LinkOut - more resources
Full Text Sources

