Phototherapy in cancer treatment: strategies and challenges
- PMID: 40169560
- PMCID: PMC11961771
- DOI: 10.1038/s41392-025-02140-y
Phototherapy in cancer treatment: strategies and challenges
Abstract
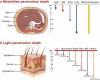
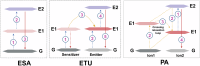
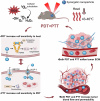
Phototherapy has emerged as a promising modality in cancer treatment, garnering considerable attention for its minimal side effects, exceptional spatial selectivity, and optimal preservation of normal tissue function. This innovative approach primarily encompasses three distinct paradigms: Photodynamic Therapy (PDT), Photothermal Therapy (PTT), and Photoimmunotherapy (PIT). Each of these modalities exerts its antitumor effects through unique mechanisms-specifically, the generation of reactive oxygen species (ROS), heat, and immune responses, respectively. However, significant challenges impede the advancement and clinical application of phototherapy. These include inadequate ROS production rates, subpar photothermal conversion efficiency, difficulties in tumor targeting, and unfavorable physicochemical properties inherent to traditional phototherapeutic agents (PTs). Additionally, the hypoxic microenvironment typical of tumors complicates therapeutic efficacy due to limited agent penetration in deep-seated lesions. To address these limitations, ongoing research is fervently exploring innovative solutions. The unique advantages offered by nano-PTs and nanocarrier systems aim to enhance traditional approaches' effectiveness. Strategies such as generating oxygen in situ within tumors or inhibiting mitochondrial respiration while targeting the HIF-1α pathway may alleviate tumor hypoxia. Moreover, utilizing self-luminescent materials, near-infrared excitation sources, non-photoactivated sensitizers, and wireless light delivery systems can improve light penetration. Furthermore, integrating immunoadjuvants and modulating immunosuppressive cell populations while deploying immune checkpoint inhibitors holds promise for enhancing immunogenic cell death through PIT. This review seeks to elucidate the fundamental principles and biological implications of phototherapy while discussing dominant mechanisms and advanced strategies designed to overcome existing challenges-ultimately illuminating pathways for future research aimed at amplifying this intervention's therapeutic efficacy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Tian, J. & Zhang, W. Synthesis, self-assembly and applications of functional polymers based on porphyrins. Prog. Polym. Sci.95, 65–117 (2019).
-
- Yanovsky, R. L. et al. Photodynamic therapy for solid tumors: a review of the literature. Photodermatol. Photoimmunol. Photomed.35, 295–303 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

