A toxin-antitoxin system provides phage defense via DNA damage and repair
- PMID: 40169592
- PMCID: PMC11961720
- DOI: 10.1038/s41467-025-58540-9
A toxin-antitoxin system provides phage defense via DNA damage and repair
Abstract
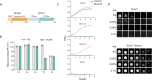
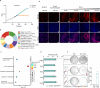
Widespread in bacteria and archaea, toxin-antitoxin (TA) systems have been recently demonstrated to function in phage defense. Here we characterize the anti-phage function of a type IV TA system, ShosTA. Using structural and biochemical approaches, we show that ShosT couples phosphoribosyltransferase and pyrophosphatase activities to disrupt purine metabolism, resulting in DNA duplication, cell filamentation and ultimate cell death. ShosA binds DNA and likely recruits other proteins to facilitate DNA homologous recombination to antagonize ShosT's toxicity. We identify Gp0.7 of T7 phage as a trigger for ShosTA system via shutting off the protein synthesis, and the C-terminus-mediated intrinsic instability of ShosA releases the toxicity of the existing ShosT proteins. Collectively, our results provide a novel toxin-antitoxin mechanism for anti-phage immunity and shed light on the triggering of this TA system.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Yamaguchi, Y., Park, J. H. & Inouye, M. Toxin-antitoxin systems in bacteria and archaea. Annu Rev. Genet45, 61–79 (2011). - PubMed
-
- Jurenas, D., Fraikin, N., Goormaghtigh, F. & Van Melderen, L. Biology and evolution of bacterial toxin-antitoxin systems. Nat. Rev. Microbiol20, 335–350 (2022). - PubMed
-
- Yarmolinsky, M. B. Programmed cell death in bacterial populations. Science267, 836–837 (1995). - PubMed
-
- Yamaguchi, Y. & Inouye, M. Regulation of growth and death in Escherichia coli by toxin-antitoxin systems. Nat. Rev. Microbiol9, 779–790 (2011). - PubMed
-
- Page, R. & Peti, W. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat. Chem. Biol.12, 208–214 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

