Intranasal influenza virus-vectored vaccine offers protection against clade 2.3.4.4b H5N1 infection in small animal models
- PMID: 40169649
- PMCID: PMC11962148
- DOI: 10.1038/s41467-025-58504-z
Intranasal influenza virus-vectored vaccine offers protection against clade 2.3.4.4b H5N1 infection in small animal models
Abstract
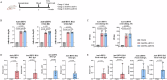
The highly pathogenic avian influenza (HPAI) H5N1 virus has been endemic in aquatic birds since 1997, causing outbreaks in domestic poultry and occasional human infections worldwide. Recently, the cross-species transmission of a new reassortant variant from clade 2.3.4.4b of H5N1 to cattle in the US has heightened concerns regarding the expansion of host range and potential human infection. As eradicating the H5N1 virus from its reservoir is impossible, it is essential to prepare for a potential pandemic caused by an H5N1 derivative. Utilizing a deleted-NS1 live attenuated influenza viral vector vaccine system (DelNS1 LAIV), a system we have previously used in the development of a COVID-19 vaccine, we have rapidly developed an intranasal vaccine for cattle H5N1 and related clade 2.3.4.4b strains, based on publicly available sequences. Our research demonstrates that a single intranasal immunization can provide effective protection against lethal challenges from HPAI cattle or mink H5N1 variants, offering strong, sustained immunity after two months in female mouse and male hamster models. Immunogenicity analysis reveals that intranasal vaccination with DelNS1 LAIV induces robust neutralizing antibody, mucosal IgA and T cell responses in mice. It is crucial to further evaluate the DelNS1-H5N1 LAIV system to prepare for potential future H5N1 outbreaks in humans.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare that the University of Hong Kong owns patents on work related to the generation and application of DelNS1 live attenuated influenza vaccines and the associated platform, with H.C., P.W. and K-Y.Y. included as co-inventors. There is no restriction on the publication of data. The other authors declare that they have no competing interests.
Figures





References
-
- Yuen, K. Y. et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet351, 467–471 (1998). - PubMed
-
- Li, K. S. et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature430, 209–213 (2004). - PubMed
-
- Chen, H. et al. Avian flu: H5N1 virus outbreak in migratory waterfowl. Nature436, 191–192 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

