Neurodegenerative and neuroinflammatory changes in SOD1-ALS patients receiving tofersen
- PMID: 40169784
- PMCID: PMC11961715
- DOI: 10.1038/s41598-025-94984-1
Neurodegenerative and neuroinflammatory changes in SOD1-ALS patients receiving tofersen
Abstract
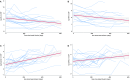
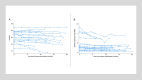
The initiation of tofersen, a new specific antisense oligonucleotide (ASO) for SOD1 pathology, marked a significant turning point for SOD1-ALS patients. While clinical trials and early access program studies reported a significant reduction in plasma and cerebrospinal fluid (CSF) neurofilament levels, neuroinflammation following prolonged treatment was never assessed. In this multicenter study, we evaluated a cohort of 18 SOD1-ALS patients treated with tofersen, analyzing correlations between biomarkers of neurodegeneration/neuroinflammation and clinical variables indicative of disease progression. NfL, NfH, CHI3L1, and Serpina1 levels in serum and CSF were determined by semi-automated immunoassays (Ella™ technology). Generalized linear mixed models were employed to investigate longitudinal trends of these biomarkers. Our data highlighted a progressive decrease in CSF neurofilament levels during tofersen treatment (MR = 0.97, 95% CI 0.94-0.99, p = 0.006 and MR = 0.98, 95% CI 0.95-1.00, p = 0.076 for NfL and NfH in CSF, respectively). Conversely, CSF levels of SerpinA1 and CHI3L1 increased over time (MR = 1.12, 95% CI 1.08-1.16, p < 0.0001 and MR = 1.039, 95% CI 1.015-1.062, p = 0.001 for SerpinA1 and CHI3L1 in CSF, respectively), but these modifications were most apparent after six and twelve months of therapy, respectively. Disease progression rate did not correlate with these biomarker trends. We observed a significant decrease in neurofilament levels during Tofersen treatment, alongside an increase in neuroinflammatory markers, potentially linked to an immune response triggered by ASO treatment. Given the limited data on tofersen's long-term efficacy in ALS due to its recent introduction, identifying biomarkers that predict clinical outcomes such as diminished therapeutic response or adverse effects is crucial. These biomarkers may help to better understand the underlying pathomechanisms of ALS and tofersen's role in modulating disease progression.
Keywords: ALS; CHI3L1; Neurofilaments; SerpinA1; Tofersen.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Each patient’s EAP for tofersen administration was approved by the local Ethical Committees of all the participating centers prior to enrolment,while this observational study received approval from the Ethics Committee of Area Vasta Emilia Nord (file number: 63/2022).
Figures
References
-
- Zou, Z.-Y. et al. Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry88, 540–549 (2017). - PubMed
-
- Arisato, T. et al. Clinical and pathological studies of familial amyotrophic lateral sclerosis (FALS) with SOD1 H46R mutation in large Japanese families. Acta Neuropathol. (Berl.)106, 561–568 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Ricerca Corrente funding scheme of the Italian Ministry of Health/Ministero della Salute
- Ricerca Finalizzata bando 2021 (RF-2021-12373036)/Ministero della Salute
- bando FAR 2021, Progetti di ricerca Interdisciplinari Mission Oriented, NEURALS project)/Università Degli Studi di Modena e Reggio Emila
- Neurobiobanca di Modena/Fondazione Cassa di Risparmio di Modena
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

