Lysyl-tRNA synthetase 1 promotes atherogenesis via autophagy-related secretion and inflammation
- PMID: 40169806
- PMCID: PMC11961584
- DOI: 10.1038/s41598-025-96046-y
Lysyl-tRNA synthetase 1 promotes atherogenesis via autophagy-related secretion and inflammation
Abstract
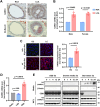
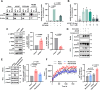
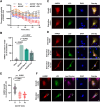
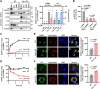
Lysyl-tRNA synthetase 1 (KARS1), an aminoacyl-tRNA synthetase, was recently identified as a secreted pro-inflammatory agent. However, the vascular secretion and functions of KARS1 have not been characterized. This study investigated the secretion mechanisms of KARS1 and explored its functional roles in vascular biology. We found that KARS1 expression was upregulated by oscillatory shear stress, an atherogenic factor, suggesting the presence of free KARS1 dissociated from aminoacyl-tRNA synthetase complexes. Moreover, in the presence of Ca2+, serum starvation triggered free cytosolic KARS1 release from endothelial cells via secretory autophagy. Both phosphatidylinositol 3-phosphate kinase and caveolin-1 were either supplementary or essential for KARS1 secretion. Secreted KARS1 co-localized in the exosome fraction of post-culture media and was externally exposed. Further, secreted KARS1 inhibited shear-induced activation of various signaling molecules, including extracellular signal-regulated kinase, protein kinase B, and endothelial nitric oxide synthetase. Secreted KARS1 in atherosclerotic plaques also acted as an atherogenic or proinflammatory autocrine/paracrine molecule. Additionally, KARS1 participated in vessel alteration. Collectively, these findings describe novel vascular features of KARS1 in response to shear stress, providing insights into shear stress-controlling mechanisms of the vascular system.
Keywords: Cell apoptosis; KARS1; Laminar shear stress; Oscillatory shear stress; Secretory autophagy; Vessel alteration.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: All animal studies in this research were approved by the Animal Use Committee of Ewha Womans University (ESM-12–0191) and Chungbuk National University (CBNUA-2242–24-02). This study was performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines. Consent for publication: All authors involved in this study have confirmed and approved the final manuscript.
Figures


References
-
- Park, S. G., Choi, E. C. & Kim, S. Aminoacyl-tRNA synthetase–interacting multifunctional proteins (AIMPs): A triad for cellular homeostasis. IUBMB Life62, 296–302 (2010). - PubMed
-
- Kovaleski, B. J. et al. In vitro characterization of the interaction between HIV-1 gag and human lysyl-tRNA synthetase. J. Bio. Chem.281, 19449–19456 (2006). - PubMed
-
- Yannay-Cohen, N. et al. LysRS serves as a key signaling molecule in the immune response by regulating gene expression. Mol. Cell34, 603–611 (2009). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

