Zebrafish Avatar testing preclinical study predicts chemotherapy response in breast cancer
- PMID: 40169839
- PMCID: PMC11961725
- DOI: 10.1038/s41698-025-00882-0
Zebrafish Avatar testing preclinical study predicts chemotherapy response in breast cancer
Abstract
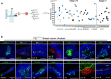
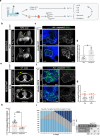
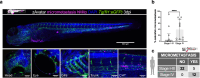
Chemotherapy remains the mainstay in most high-risk breast cancer (BC) settings, with several equivalent options of treatment. However, the efficacy of each treatment varies between patients and there is currently no test to determine which option will be the most effective for each individual patient. Here, we developed a fast in-vivo test for BC therapy screening: the zebrafish patient-derived-xenograft model (zAvatars), where in-vivo results can be obtained in just 10 days. To determine the predictive value of the BC zAvatars we performed a preclinical study, where zAvatars were treated with the same therapy as the donor-patient and their response to therapy was compared. Our data show a 100% concordance (18 out of 18) between the zAvatar-test and the corresponding patient's clinical response to treatment. Altogether, our results suggest that the zAvatar model constitutes a promising in-vivo assay to optimize cancer treatments in a truly personalized manner.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Harbeck, N. et al. Breast cancer. Nat. Rev. Dis. Prim.5, 66 (2019). - PubMed
-
- Perou, C. M. et al. Molecular portraits of human breast tumours. Nature406, 747–752 (2000). - PubMed
-
- Geyer, F. C. et al. Molecular analysis reveals a genetic basis for the phenotypic diversity of metaplastic breast carcinomas. J. Pathol.220, 562–573 (2010). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

