An inflammatory biomarker signature of response to CAR-T cell therapy in non-Hodgkin lymphoma
- PMID: 40169864
- PMCID: PMC12003198
- DOI: 10.1038/s41591-025-03532-x
An inflammatory biomarker signature of response to CAR-T cell therapy in non-Hodgkin lymphoma
Abstract
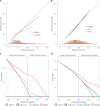
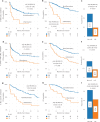
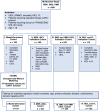
Disease progression is a substantial challenge in patients with non-Hodgkin lymphoma (NHL) undergoing chimeric antigen receptor T cell (CAR-T) therapy. Here we present InflaMix (INFLAmmation MIXture Model), an unsupervised quantitative model integrating 14 pre-CAR-T infusion laboratory and cytokine measures capturing inflammation and end-organ function. Developed using a cohort of 149 patients with NHL, InflaMix revealed an inflammatory signature associated with a high risk of CAR-T treatment failure, including increased hazard of death or relapse (hazard ratio, 2.98; 95% confidence interval, 1.60-4.91; P < 0.001). Three independent cohorts comprising 688 patients with NHL from diverse treatment centers were used to validate our approach. InflaMix consistently and reproducibly identified patients with a higher likelihood of disease relapse and mortality, and it provided supplementary predictive value beyond established prognostic markers, including tumor burden. Moreover, InflaMix exhibited robust performance in cases with missing data, maintaining accuracy when considering only six readily available laboratory measures. These findings show that InflaMix is a valuable tool for point-of-care clinical decision-making in patients with NHL undergoing CAR-T therapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: R.S. reports speaker honorarium from Incyte. A.A. reports honoraria from AbbVie and has consulting and advisory roles with Takeda, Gilead, Novartis, Roche and Bristol Myers Squibb. L.A.L. served as a consultant and/or speaker bureau for Kite/Gilead, Beigene, Pharmacyclics, AbbVie, Genmab, SeaGen, Janssen, AstraZeneca, Eli Lilly, Epizyme, TG Therapeutics, Merck and ADC Therapeutics. A.I. owns stock and has ownership interests in Cota Healthcare. He also reports honoraria from MJH Life Sciences and Pfizer. He served as a consultant and/or speaker bureau for TG Therapeutics, Secura Bio, AstraZeneca and Seattle Genetics. Z.E.-P. serves on Genmab advisory board. J.U.P. reports research funding, intellectual property fees and travel reimbursement from Seres Therapeutics and consulting fees from Da Volterra, CSL Behring and MaaT Pharma. He serves on an advisory board of and holds equity in Postbiotics Plus Research. He has filed intellectual property applications related to the microbiome (reference numbers #62/843,849, #62/977,908 and #15/756,845). R.J.L. has served as a consultant for Kite. G.L.S. has received research funding from Janssen, Amgen, BMS, Beyond Spring, GPCR and Recordati and serves on the DSMB for Arcellx. M.S. served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc., and Omeros Corporation; received research funding from Angiocrine Bioscience, Inc., Omeros Corporation, Amgen Inc., Bristol Myers Squibb, and Sanofi; served on ad hoc advisory boards for Kite – A Gilead Company, and Miltenyi Biotec; and received honoraria from i3Health, Medscape, CancerNetwork, Intellisphere LLC, Curio Science LLC, and IDEOlogy. S.A.G. receives research funding from Amgen, Johnson & Johnson, Takeda, Celgene, Actinium, Sanofi, Miltenyi, Kite and EUSA. He is on the advisory boards of Amgen, Johnson & Johnson, Takeda, Celgene, Actinium, Sanofi, Miltenyi, Novartis, Kite, Jazz, BMS, Spectrum Pharma and EUSA Omeros. J.H.P. received consulting fees from Affyimmune Therapeutics, Amgen, Autolus, Be Biopharma, Beigene, Bright Pharmaceutical Services, Curocel, Kite, Medpace, Minerva Biotechnologies, Pfizer, Servier, Sobi and Takeda; received honoraria from OncLive, Physician Education Resource and MJH Life Sciences; serves on scientific advisory board of Allogene Therapeutics and Artiva Biotherapeutics; and received institutional research funding from Autolus, Genentech, Fate Therapeutics, Incyte, Servier and Takeda. M.L.P. has served as a consultant for Novartis, Cellectar, Synthekine, Kite, Seres, Magenta, WindMIL, Rheos, Nektar, Notch, Priothera, Ceramedix, Lygenesis and Pluto. G.S. has received in the last 12 months financial compensation for participating in advisory boards or consulting from AbbVie, Atbtherapeutics, Beigene, BMS/Celgene, Debiopharm, Genentech/Roche, Genmab, Incyte, Ipsen, Janssen, Kite/Gilead, Loxo/Lilly, Merck, Molecular Partners, Nordic Nanovector, Novartis, Nurix and Orna. He has also received research support managed by his institution from Genentech, Janssen and Ipsen. He is a shareholder of Owkin. B.D.G. has received honoraria for speaking engagements from Merck, Bristol Myers Squibb and Chugai Pharmaceuticals; has received research funding from Bristol Myers Squibb and Merck; and has been a compensated consultant for Darwin Health, Merck, PMV Pharma, Shennon Biotechnologies and Rome Therapeutics, of which he is a co-founder. He additionally has intellectual property rights with Rome Therapeutics and the Icahn School of Medicine at Mount Sinai. He has served in an advisory role at Merck Sharpe and Dohme and Darwin Health. M.-A.P. reports honoraria from Adicet, Allogene, Allovir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG and Vor Biopharma. He serves on DSMBs for Cidara Therapeutics, Medigene and Sellas Life Sciences and the scientific advisory board of NexImmune. He has ownership interests in NexImmune, Omeros and OrcaBio. He has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. M.R.M.v.d.B. has received research support and stock options from Seres Therapeutics and stock options from Notch Therapeutics and Pluto Therapeutics; he has received royalties from Wolters Kluwer; has consulted, received honorarium from or participated in advisory boards for Seres Therapeutics, Vor Biopharma, Rheos Medicines, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Ceramedix, Lygenesis, Pluto Therapeutics, GlaxoSmithKline, Da Volterra, Thymofox, Garuda, Novartis (spouse), Synthekine (spouse), Beigene (spouse) and Kite (spouse); he has IP licensing with Seres Therapeutics and Juno Therapeutics; and holds a fiduciary role on the Foundation Board of DKMS (a nonprofit organization). Memorial Sloan Kettering Cancer Center has institutional financial interests relative to Seres Therapeutics. The other authors declare no competing interests. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

