Efficacy and safety of belumosudil for treatment of cGVHD: multicenter retrospective analysis of the French cohort of the compassionate use program, on behalf of the French Society of Bone Marrow Transplantation and Cellular Therapy
- PMID: 40169928
- PMCID: PMC12151857
- DOI: 10.1038/s41409-025-02554-w
Efficacy and safety of belumosudil for treatment of cGVHD: multicenter retrospective analysis of the French cohort of the compassionate use program, on behalf of the French Society of Bone Marrow Transplantation and Cellular Therapy
Abstract
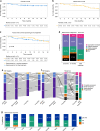
Chronic graft versus host disease is a major cause of morbidity after allogeneic haematopoietic cell transplantation. Belumosudil has recently been approved for the treatment of cGVHD refractory after two lines of treatment. However, few data are available to evaluate its efficacy and safety in real life. 68 patients with cGVHD received belumosudil through a compassionate access program in France. The median follow-up was 337 days from belumosudil initiation. Eighty-two percent of patients had severe cGVHD with a median of three organs involved. Patients had received a median of three prior treatment lines. Median treatment duration was 251 days. The best overall response rate (ORR) was 57.3%, including 14.7% complete remission (CR) and 42.6% partial response (PR). The ORR at three and six months was 47% and 45.6%, respectively. Liver and mouth involvement showed the highest response rates (72.7% and 70.4%), while lung involvement had the lowest (17.2%). Median failure-free survival (FFS) was not reached, with 6- and 12-month FFS rates of 89.1% and 80.4%, respectively. Nine patients died, mainly from GVHD (n = 5). Ten adverse events were reported, leading to treatment discontinuation in three cases. These results support the efficacy and safety of belumosudil in refractory cGVHD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: DM received research grants from Novartis, Sanofi and CSL Behring, consulting fees from Novartis, Incyte, Sanofi, Jazz Pharmaceuticals, and Mallinckrodt. FM received honoraria from Therakos/Mallinckrodt, Janssen, Biocodex, Sanofi, JAZZ Pharmaceuticals, Gilead, Novartis, Priothera, and Astellas. EF received speaker fees and honoraria for advisory board from Novartis, Sanofi, Gilead, Alexion, MSD, Astellas, Jazz, Pfizer, GSK and Sobi. ML received research grant from Janssen, consulting honoraria from Abbvie, Alexion, Bristol Myers Squibb, Gilead, Glaxo Smith Kline, Incyte, Jazz, Kartos, Mallinkrodt, Novartis, Roche, Sanofi, Takeda. Other authors did not declare any competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

