Assessing user experience with the Bioline™ HCV point-of-care test in primary healthcare settings: a mixed-methods study
- PMID: 40170035
- PMCID: PMC11963430
- DOI: 10.1186/s12913-025-12634-8
Assessing user experience with the Bioline™ HCV point-of-care test in primary healthcare settings: a mixed-methods study
Abstract
Background: Hepatitis C Virus (HCV) is a major public health challenge, particularly in resource-limited settings with inadequate diagnostic services. The Bioline™ HCV Point-of-Care (POC) test provides a promising solution for improving diagnosis in Primary Healthcare (PHC) clinics without laboratory infrastructure. This study evaluated the test's usability, acceptability, and deliverability in Ghana using user-oriented REASSURED criteria.
Methods: A convergent parallel mixed-methods design was adopted. Quantitative data was collected through direct observation of Healthcare Workers (HCWs) using audit checklists and analyzed with Stata 16. The analysis included descriptive statistics, inter-rater concordance assessment, and the application of the System Usability Scale (SUS). Qualitative data, analyzed using Atlas.ti 24.2.0, explored user experiences, confidence, storage infrastructure, and suggestions for test design improvement through in-depth interviews.
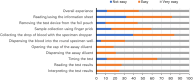
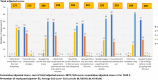
Results: The quantitative audit included 81 non-laboratory HCWs, with 22 participating in in-depth interviews. The test scored 88.7 on the SUS (95% CI: 86.40-90.88), with 88% of HCWs rating it as easy or very easy to use. Most HCWs (81.5%) successfully completed all testing steps independently, achieving 100% inter-rater concordance, but 83% made errors in at least one step, primarily during pre-testing. Qualitative findings revealed widespread acceptance, confidence, and adaptability despite challenges with storage infrastructure.
Discussion: The Bioline™ HCV POC test demonstrated high usability and acceptance among HCWs in resource-limited settings. Enhancements such as improved packaging, simplified information sheets, refined droppers, and additional components like gloves could further optimize usability. These findings support the Sustainable Development Goal (SDG) 3 by enhancing access to timely HCV diagnosis, contributing to Universal Health Coverage, and strengthening health systems in underserved areas.
Trial registration: This study is part of a diagnostic trial registered in the Pan African Clinical Trial Registry ( https://pactr.samrc.ac.za ) on 24th October 2024 with trial registration number: PACTR202410837698664.
Keywords: Acceptability; Primary healthcare; Usability; User-experience, UX.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study received full approval from the Faculty of Health Sciences Research Ethics Committee, University of Pretoria, South Africa (281/2023). In addition, the Ghana Health Service Ethics Review Committee approved the study protocol and granted permission to use the PHC clinics in Ghana (GHS-ERC013/08/23). Introductory and approval letters from the Ghana Health Service were sent to all the PHC clinics included in the study prior to its commencement. Informed consent was obtained from all participating HCWs before they were deemed eligible for inclusion in the study. The study methods adhered to strict ethical guidelines and regulations in accordance with the Helsinki declaration. Consent for publication: Not applicable. Competing interests: EMM declares that he is employed by Abbott Laboratories as a Scientific Affairs Manager for Africa and a co-supervisor to ED. He, therefore, acknowledges that he is aware of his responsibility to take the necessary steps to take reasonable steps to avoid any potential or perceived conflict of interest during his co-supervision duties in this study. Moreover, as a co-supervisor, his contribution and decisions were reviewed and approved by TM-T, the main supervisor of the study. All authors declare no other conflict of interest.
Figures
Similar articles
-
REASSURED evaluation of the Bioline HCV point-of-care testing for diagnosing hepatitis C virus infection in primary healthcare settings of Ghana: a study protocol.BMJ Open. 2024 Nov 9;14(11):e082416. doi: 10.1136/bmjopen-2023-082416. BMJ Open. 2024. PMID: 39521466 Free PMC article.
-
Poor supply chain management and stock-outs of point-of-care diagnostic tests in Upper East Region's primary healthcare clinics, Ghana.PLoS One. 2019 Feb 27;14(2):e0211498. doi: 10.1371/journal.pone.0211498. eCollection 2019. PLoS One. 2019. PMID: 30811407 Free PMC article.
-
Usability and acceptability of self-testing for hepatitis C virus exposure in a high-prevalence urban informal settlement in Karachi, Pakistan.BMC Infect Dis. 2024 Sep 27;24(1):1054. doi: 10.1186/s12879-024-09925-6. BMC Infect Dis. 2024. PMID: 39333922 Free PMC article.
-
Seroprevalence of viral hepatitis B and C infections among healthcare workers in Ethiopia: A systematic review and meta-analysis.PLoS One. 2024 Nov 7;19(11):e0312959. doi: 10.1371/journal.pone.0312959. eCollection 2024. PLoS One. 2024. PMID: 39509362 Free PMC article.
-
The Urgent Need to Implement Point-of-Care RNA Testing for Hepatitis C Virus to Support Elimination.Clin Infect Dis. 2024 May 15;78(5):1235-1239. doi: 10.1093/cid/ciad503. Clin Infect Dis. 2024. PMID: 37633653 Free PMC article. Review.
References
-
- WHO. Hepatitis C. World Health Organization. 2023. [cited 2023 Sep 2]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
-
- WHO. Hepatitis: disease burden. World Health Oganisation. 2021. Available from: https://www.afro.who.int/health-topics/hepatitis.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical