Rapid and visual detection of transmissible gastroenteritis virus using a CRISPR/Cas12a system combined with loop-mediated isothermal amplification
- PMID: 40170086
- PMCID: PMC11963519
- DOI: 10.1186/s12917-025-04711-1
Rapid and visual detection of transmissible gastroenteritis virus using a CRISPR/Cas12a system combined with loop-mediated isothermal amplification
Abstract
Background: Transmissible gastroenteritis (TGE) is a highly contagious intestinal disease caused by transmissible gastroenteritis virus (TGEV). The primary techniques for identifying TGEV involve enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction (PCR), and fluorescent quantitative PCR (qPCR). However, these approaches are complex, demanding specialized tools and significant time. Therefore, a precise, swift, and effective differential diagnosis method is crucial for TGEV prevention. In recent years, clustered regularly interspaced short palindromic repeats (CRISPR) and Cas-associated proteins have become popular for their high specificity, unique cleavage activity, and ease of detection. CRISPR-Cas12a, a novel RNA-guided nucleic acid endonuclease, is emerging as a powerful molecular scissor.
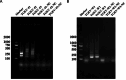
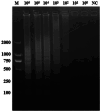
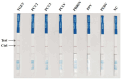
Results: In this study, we designed three pairs of crRNA targeting the N gene of TGEV. Following the selection of the most appropriate crRNA, we established the loop-mediated isothermal (LAMP) amplification method with a sensitivity of 102 copies/µL. And based on this, we established the CRISPR-Cas12a fluorescence assay with a sensitivity of 100 copies/µL. Furthermore, we established a CRISPR/Cas12a lateral-flow dipstick assay with a sensitivity of 102 copies/µL. Importantly, none of these methods exhibited cross-reactivity with other related viruses, enabling quicker and more straightforward observation of experimental results.
Conclusions: We have successfully developed a CRISPR-Cas12a fluorescence assay and a CRISPR/Cas12a lateral-flow dipstick assay for clinical TGEV detection. Overall, we created a portable, quick, and sensitive TGEV assay with strong specificity utilizing the CRISPR-Cas12a system.
Keywords: CRISPR-Cas12a; Detection; LAMP; TGEV.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

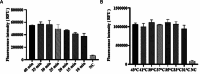






Similar articles
-
Development of a triplex RT-RAA-LFA assay for the rapid differential diagnosis of porcine epidemic diarrhea virus, porcine deltacoronavirus and transmissible gastroenteritis virus.Microb Pathog. 2024 Oct;195:106885. doi: 10.1016/j.micpath.2024.106885. Epub 2024 Aug 24. Microb Pathog. 2024. PMID: 39182857
-
Fast-Flu: RT-RPA-CRISPR/Cas12a assisted one-step platform for rapid influenza B virus detection.Microbiol Spectr. 2025 Jun 3;13(6):e0036525. doi: 10.1128/spectrum.00365-25. Epub 2025 Apr 25. Microbiol Spectr. 2025. PMID: 40277382 Free PMC article.
-
Reverse transcription loop-mediated isothermal amplification for rapid detection of transmissible gastroenteritis virus.Curr Microbiol. 2011 Mar;62(3):1074-80. doi: 10.1007/s00284-010-9825-9. Epub 2010 Dec 2. Curr Microbiol. 2011. PMID: 21127872 Free PMC article.
-
Detection of Porcine Circovirus (PCV) Using CRISPR-Cas12a/13a Coupled with Isothermal Amplification.Viruses. 2024 Sep 30;16(10):1548. doi: 10.3390/v16101548. Viruses. 2024. PMID: 39459882 Free PMC article. Review.
-
An overview of immunological and genetic methods for detecting swine coronaviruses, transmissible gastroenteritis virus, and porcine respiratory coronavirus in tissues.Adv Exp Med Biol. 1997;412:37-46. doi: 10.1007/978-1-4899-1828-4_4. Adv Exp Med Biol. 1997. PMID: 9191988 Review.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

