Nuciferine Attenuates Cancer Cachexia-Induced Muscle Wasting in Mice via HSP90AA1
- PMID: 40170230
- PMCID: PMC11961380
- DOI: 10.1002/jcsm.13777
Nuciferine Attenuates Cancer Cachexia-Induced Muscle Wasting in Mice via HSP90AA1
Abstract
Background: Around 80% of patients with advanced cancer have cancer cachexia (CC), a serious complication for which there are currently no FDA-approved treatments. Nuciferine (NF) is the main active ingredient of lotus leaf, which has anti-inflammatory, anti-tumour and other effects. The purpose of this work was to explore the target and mechanism of NF in preventing cancer cachexia-induced muscle atrophy.
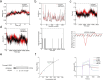
Methods: The action of NF against CC-induced muscle atrophy was determined by constructing an animal model with a series of behavioural tests, H&E staining and related markers. Network pharmacology and molecular docking were used to preliminarily determine the mechanism and targets of NF against CC-induced muscle atrophy. The mechanisms of NF in treating CC-induced muscle atrophy were verified by western blotting. Molecular dynamics simulation (MD), drug affinity responsive target stability (DARTS) and surface plasmon resonance (SPR) were used to validate the key target of NF.
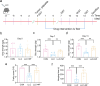
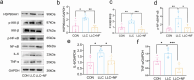
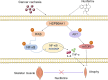
Results: After 13 days of NF treatment, the reduction of limb grip strength and hanging time in LLC model mice increased by 29.7% and 192.2% (p ≤ 0.01; p ≤ 0.001). Gastrocnemius and quadriceps muscles weight/initial body weight (0.98 ± 0.11 and 1.20 ± 0.17) and cross-sectional area of muscle fibres (600-1600 μm2) of NF-treated mice were significantly higher than those of the model group (0.84 ± 0.10, 0.94 ± 0.09, 400-800 μm2, respectively) (p ≤ 0.01; p ≤ 0.01; p ≤ 0.001). NF treatment also decreased the MyHC (myosin heavy chain) degradation and the protein levels of muscle-specific E3 ubiquitin ligases Atrogin1 and MuRF1 in the model group (p ≤ 0.001; p ≤ 0.01; p ≤ 0.05). Network pharmacology revealed that NF majorly targeted AKT1, TNF and HSP90AA1 to regulate PI3K-Akt and inflammatory pathways. Molecular docking predicted that NF bound best to HSP90AA1. Mechanism analysis demonstrated that NF regulated NF-κB and AKT-mTOR pathways for alleviating muscle wasting in tumour bearing mice. The results of MD, DARTS and SPR further confirmed that HSP90AA1 was the direct target of NF.
Conclusions: Overall, we first discovered that NF retards CC-induced muscle atrophy by regulating AKT-mTOR and NF-κB signalling pathways through directly binding HSP90AA1, suggesting that NF may be an effective treatment for cancer cachexia.
Keywords: AKT–mTOR; HSP90AA1; NF‐κB; cancer cachexia, muscle wasting; nuciferine.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Huang Q., Wu M., Wu X., Zhang Y., and Xia Y., “Muscle‐To‐Tumor Crosstalk: The Effect of Exercise‐Induced Myokine on Cancer Progression,” Biochimica et Biophysica Acta (BBA) ‐ Reviews on Cancer 1877 (2022): 188761. - PubMed
-
- Wang Y., Sun X., Yang Q., and Guo C., “Cucurbitacin IIb Attenuates Cancer Cachexia Induced Skeletal Muscle Atrophy by Regulating the IL‐6/STAT3/FoxO Signaling Pathway,” Phytotherapy Research 37 (2023): 3380–3393. - PubMed
-
- Cohen S., Nathan J. A., and Goldberg A. L., “Muscle Wasting in Disease: Molecular Mechanisms and Promising Therapies,” Nature Reviews. Drug Discovery 14 (2015): 58–74. - PubMed
-
- Bilgic S. N., Domaniku A., Toledo B., et al., “EDA2R‐NIK Signalling Promotes Muscle Atrophy Linked to Cancer Cachexia,” Nature 617 (2023): 827–834. - PubMed
MeSH terms
Substances
Grants and funding
- 82360829/National Natural Science Foundation of China
- Research Foundation of Xinjiang Uygur Autonomous Region Introduction plan of 'Tianchi Talents'
- 2022ZK020/Shihezi University High-Level Talents' Research Initiation Project
- K202212/Open Funds for the State Key Laboratory of Natural and Biomimetic Drugs
- Doctoral Scientific Research Foundation of Shihezi University College of Pharmacy
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

