Effect of Cladribine Tablets on the Pharmacokinetics of a Combined Oral Contraceptive in Pre-Menopausal Women With Relapsing Multiple Sclerosis
- PMID: 40170281
- PMCID: PMC11961393
- DOI: 10.1111/cts.70204
Effect of Cladribine Tablets on the Pharmacokinetics of a Combined Oral Contraceptive in Pre-Menopausal Women With Relapsing Multiple Sclerosis
Abstract
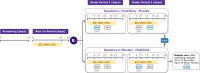
This study assessed the effect of cladribine tablets (CladT) on the pharmacokinetics (PK) of a combined oral contraceptive (COC) in pre-menopausal women with relapsing multiple sclerosis. It was a randomized, double-blind, two-period, two-sequence crossover study to assess steady-state plasma PK (area under the concentration-time curve and peak concentration) of COC (ethinylestradiol [EE] 30 μg and levonorgestrel [LNG] 150 μg) when co-administered with CladT or placebo. Participants received 2 weeks of active CladT treatment per course (Weeks 1 and 5 per year) to have a cumulative dose of 3.5 mg/kg over 2 years as per label. Of the 24 randomized participants, 23 completed the study. The results showed that the concentration-time profiles as well as PK parameters of EE and LNG in the plasma were similar when co-administered with CladT or placebo. Analysis of variance confirmed the bioequivalence of EE and LNG in COC when co-administered with either CladT or placebo. All participants were adequately exposed to cladribine. Repeat-dose administration of CladT had no apparent effect on serum luteinizing hormone, follicle-stimulating hormone, progesterone, or sex hormone-binding globulin concentrations during concomitant treatment with COC. Co-administration with COC did not change the known safety and tolerability profile of CladT and did not alter the PK of EE or LNG in a COC during the study. Therefore, the concomitant use of CladT is not expected to decrease the efficacy of COCs containing EE and LNG. Trial Registration: EudraCT Number: 2018-001015-70.
Keywords: cladribine tablets; drug–drug interaction; oral contraceptives; pharmacokinetics; relapsing multiple sclerosis.
© 2025 EMD Serono and The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
R.H. is an independent Clinical Pharmacology consultant and served as an external Clinical Pharmacology expert advisor for various aspects in the clinical development of Cladribine. He received financial support for research, consulting, and training services from the healthcare business of Merck KGaA, Darmstadt, Germany. K.H. has received speaker honoraria and research support from Bayer, Biogen, the healthcare business of Merck KGaA, Darmstadt, Germany, Novartis, Sanofi Genzyme, Roche, and Teva; has received support for congress participation from Bayer, Biogen, the healthcare business of Merck KGaA, Darmstadt, Germany, Roche, Sanofi Genzyme, and Teva; and has served on scientific advisory boards for Bayer, Biogen, Sanofi, Teva, Roche, Novartis, and the healthcare business of Merck KGaA, Darmstadt, Germany. S.G., A.B., A.K.‐B., C.V., and A.N. are employees of the healthcare business of Merck KGaA, Darmstadt, Germany. A.G. was an employee and is now a consultant to Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany. D.J. is an employee of Merck Serono Ltd., Feltham, UK, an affiliate of Merck KGaA, Darmstadt, Germany. K.V. and J.Q.D. are employees of EMD Serono, Billerica, MA, USA.
Figures

References
-
- The Multiple Sclerosis International Federation , Atlas of MS, 3rd ed. (Multiple Sclerosis International Federation, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

