Dolutegravir plus lamivudine downregulates cellular stress responses vs. three-drug HIV regimens
- PMID: 40170606
- PMCID: PMC12237123
- DOI: 10.1097/QAD.0000000000004198
Dolutegravir plus lamivudine downregulates cellular stress responses vs. three-drug HIV regimens
Abstract
Objective: To compare the systemic and immune effects of two-drug regimens (2DR) and three-drug regimens (3DR) in people with HIV (PWH).
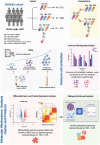
Design: In a cross-sectional study, multiomics data were analyzed in dolutegravir (DTG) plus lamivudine (3TC) 2DR and 3DR comprising DTG with two nucleoside reverse transcriptase inhibitors (NRTIs).
Methods: Data from the 2000HIV cohort of virally suppressed PWH on combination antiretroviral treatment (cART) regimens were analyzed. Groups included DTG + 3TC ( n = 191), DTG + 3TC + abacavir (ABC) ( n = 188), and DTG + tenofovir disoproxil fumarate or tenofovir alafenamide (TDF/TAF) + emtricitabine (FTC) ( n = 115). Systemic functions were assessed via plasma protein profiling (Olink Explore, 2367 proteins), while peripheral blood mononuclear cells (PBMCs) were used to evaluate immune effects by analyzing Bulk RNA-Seq and ex-vivo cytokine production capacity.
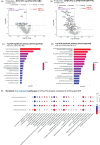
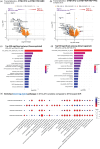
Results: Plasma protein analysis revealed that 2DR associated to lower protein expression and pathways related to metabolism, stress responses, chemokine signaling, and immune responses compared to 3DR. Four proteins - CXCL8, DDC, PMM2, and EPS8L2 - were consistently downregulated in 2DR. Differential gene expression analysis identified 17 overlapping downregulated genes across all 3DR vs. 2DR comparisons, linked to chromatin structure, cellular senescence, stress response, and cytokine activity. Cytokine production was similar across 2DR and 3DR groups, except for enhanced interleukin (IL)-17 production in DTG + TDF + FTC users.
Conclusions: Reducing NRTIs in DTG-based 2DR, particularly by omitting ABC or TAF/TDF, suggests decreased activation of stress response and immune-related pathways. Importantly, the functional capacity of circulating immune cells remains largely unchanged between 2DR and 3DR.
Keywords: HIV; antiretroviral therapy; dolutegravir; multiomics; reverse transcriptase inhibitors.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
All authors are part of the 2000HIV collaboration, supported by ViiV Healthcare. This research was funded by ViiV Healthcare. However, ViiV Healthcare had no involvement in data quality control, statistical analyses, or the final interpretation of the results.
Figures


References
-
- Seyler L, Lacor P, Allard SD. Current challenges in the treatment of HIV. Pol Arch Intern Med 2018; 128:609–616. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV; 2024.
-
- Venter WDF, Sokhela S, Simmons B, Moorhouse M, Fairlie L, Mashabane N, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, noninferiority trial. Lancet HIV 2020; 7:e666–e676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

