A Self-Assembled Metabolic Regulator Reprograms Macrophages to Combat Cytokine Storm and Boost Sepsis Immunotherapy
- PMID: 40171016
- PMCID: PMC11959697
- DOI: 10.34133/research.0663
A Self-Assembled Metabolic Regulator Reprograms Macrophages to Combat Cytokine Storm and Boost Sepsis Immunotherapy
Abstract
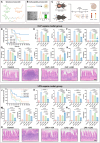
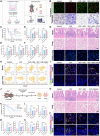
Sepsis, a life-threatening inflammatory disorder characterized by multiorgan failure, arises from a dysregulated immune response to infection. Modulating macrophage polarization has emerged as a promising strategy to control sepsis-associated inflammation. The endogenous metabolite itaconate has shown anti-inflammatory potential by suppressing the stimulator of interferon genes (STING) pathway, but its efficacy is inhibited by hyperactive glycolysis, which sustains macrophage overactivation. Here, we revealed a critical crosstalk between the itaconate-STING axis and glycolysis in macrophage-mediated inflammation. Building on this interplay, we developed a novel nanoparticle LDO (lonidamine disulfide 4-octyl-itaconate), a self-assembled metabolic regulator integrating an itaconate derivative with the glycolysis inhibitor Lonidamine. By concurrently targeting glycolysis and STING pathways, LDO reprograms macrophages to restore balanced polarization. In sepsis models, LDO effectively attenuates CCL2-driven cytokine storms, alleviates acute lung injury, and significantly enhances survival via metabolic reprogramming. This study offers a cytokine-regulatory strategy rooted in immunometabolism, providing a foundation for the translational development of immune metabolite-based sepsis therapies.
Copyright © 2025 Junyan Zhuang et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures







References
-
- Beane A, Shankar-Hari M. Long-term ill health in sepsis survivors: An ignored health-care challenge? Lancet. 2024;404(10459):1178–1180. - PubMed
-
- Kabat AM, Pearce EJ. Inflammation by way of macrophage metabolism. Science. 2017;356(6337):488–489. - PubMed
-
- Hegarty LM, Jones G-R, Bain CC. Macrophages in intestinal homeostasis and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2023;20(8):538–553. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

