Effects of aged garlic extract on aging?related changes in gastrointestinal function and enteric nervous system cells
- PMID: 40171138
- PMCID: PMC11959352
- DOI: 10.3892/etm.2025.12853
Effects of aged garlic extract on aging?related changes in gastrointestinal function and enteric nervous system cells
Abstract
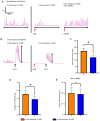
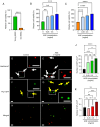
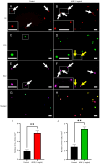
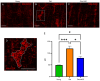
Dysmotility of the gastrointestinal (GI) tract is commonly seen in elderly individuals, where it causes significant morbidity and can lead to more severe conditions, including sarcopenia and frailty. Although the precise mechanisms underlying aging-related GI dysmotility are not fully understood, neuronal loss or degeneration in the enteric nervous system (ENS) may be involved. Aged garlic extract (AGE) has been shown to have several beneficial effects in the GI tract; however, it is not known whether AGE can improve GI motility in older animals. The aim of the present study was to examine the effects of AGE on the ENS and gut motility in older mice and elucidate potential mechanisms of action. An AGE-formulated diet was given to 18-month-old female mice for 2 weeks. Organ bath studies and cell culture demonstrated that AGE: i) Altered gut contractile activity; ii) enhanced viability of ENS cells; and iii) exhibited neuroprotective effects on the ENS via reduction in oxidative stress. These findings suggest that AGE could be used to develop novel dietary therapeutics for aging-related GI dysmotility by targeting the associated loss and damage of the ENS.
Keywords: aged garlic extract; aging; enteric nervous system; intestinal motility; neuronal nitric oxide synthase; neuroprotection; oxidative stress.
Copyright: © 2025 Ohishi et al.
Conflict of interest statement
The authors declare that they have competing interests: the work was funded by Wakunaga Pharmaceutical Company Ltd., where KO is an employee.
Figures


Similar articles
-
Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia.Microbiome. 2021 Oct 26;9(1):210. doi: 10.1186/s40168-021-01165-z. Microbiome. 2021. PMID: 34702353 Free PMC article.
-
Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression.Gastroenterology. 2019 Aug;157(2):507-521.e4. doi: 10.1053/j.gastro.2019.04.022. Epub 2019 May 7. Gastroenterology. 2019. PMID: 31071306 Free PMC article.
-
Novel understanding on genetic mechanisms of enteric neuropathies leading to severe gut dysmotility.Eur J Histochem. 2021 Nov 25;65(s1):3289. doi: 10.4081/ejh.2021.3289. Eur J Histochem. 2021. PMID: 34818877 Free PMC article. Review.
-
Altered gastrointestinal motility involving autoantibodies in the experimental autoimmune encephalomyelitis model of multiple sclerosis.Neurogastroenterol Motil. 2018 Sep;30(9):e13349. doi: 10.1111/nmo.13349. Epub 2018 Apr 11. Neurogastroenterol Motil. 2018. PMID: 29644797 Free PMC article.
-
Aging and neural control of the GI tract. I. Age-related changes in the enteric nervous system.Am J Physiol Gastrointest Liver Physiol. 2002 Sep;283(3):G489-95. doi: 10.1152/ajpgi.00091.2002. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 12181159 Review.
References
LinkOut - more resources
Full Text Sources
