Divergent host humoral innate immune response to the smooth-to-rough adaptation of Mycobacterium abscessus in chronic infection
- PMID: 40171164
- PMCID: PMC11959001
- DOI: 10.3389/fcimb.2025.1445660
Divergent host humoral innate immune response to the smooth-to-rough adaptation of Mycobacterium abscessus in chronic infection
Abstract
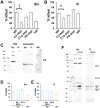
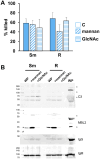
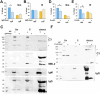
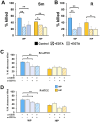
Mycobacterium abscessus is a nontuberculous mycobacterium emerging as a significant pathogen in individuals with chronic lung diseases, including cystic fibrosis and chronic obstructive pulmonary disease. Current therapeutics have poor efficacy. Strategies of bacterial control based on host defenses are appealing; however, antimycobacterial immunity remains poorly understood and is further complicated by the appearance of smooth and rough morphotypes, which elicit distinct host responses. We investigated the role of serum components in neutrophil-mediated clearance of M. abscessus morphotypes. M. abscessus opsonization with complement enhanced bacterial killing compared to complement-deficient opsonization. Killing of rough isolates was less reliant on complement. Complement C3 and mannose-binding lectin 2 (MBL2) were deposited on M. abscessus morphotypes in distinct patterns, with a greater association of MBL2 on rough M. abscessus. Killing was dependent on C3; however, depletion and competition experiments indicate that canonical complement activation pathways are not involved. Complement-mediated killing relied on natural IgG and IgM for smooth morphotypes and on IgG for rough morphotypes. Both morphotypes were recognized by complement receptor 3 in a carbohydrate- and calcium-dependent manner. These findings indicate a role for noncanonical C3 activation pathways for M. abscessus clearance by neutrophils and link smooth-to-rough adaptation to complement activation.
Keywords: adaptation; complement; cystic fibrosis; natural antibodies; neutrophils.
Copyright © 2025 Wheeler, Lenhart-Pendergrass, Rysavy, Poch, Caceres, Calhoun, Serban, Nick and Malcolm.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Update of
-
Divergent host innate immune response to the smooth-to-rough M. abscessus adaptation to chronic infection.bioRxiv [Preprint]. 2023 May 16:2023.05.15.540822. doi: 10.1101/2023.05.15.540822. bioRxiv. 2023. Update in: Front Cell Infect Microbiol. 2025 Mar 18;15:1445660. doi: 10.3389/fcimb.2025.1445660. PMID: 37293112 Free PMC article. Updated. Preprint.
References
-
- Bhakdi S., Knufermann H., Fischer H., Wallach D. F. (1974). Interaction between erythrocyte membrane proteins and complement components. II. The identification and peptide composition of complement components C3 and C4 desorbed from erythrocyte membranes. Biochim. Biophys. Acta 373, 295–307. doi: 10.1016/0005-2736(74)90153-9 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

