Utilizing large language models for gastroenterology research: a conceptual framework
- PMID: 40171241
- PMCID: PMC11960180
- DOI: 10.1177/17562848251328577
Utilizing large language models for gastroenterology research: a conceptual framework
Abstract
Large language models (LLMs) transform healthcare by assisting clinicians with decision-making, research, and patient management. In gastroenterology, LLMs have shown potential in clinical decision support, data extraction, and patient education. However, challenges such as bias, hallucinations, integration with clinical workflows, and regulatory compliance must be addressed for safe and effective implementation. This manuscript presents a structured framework for integrating LLMs into gastroenterology, using Hepatitis C treatment as a real-world application. The framework outlines key steps to ensure accuracy, safety, and clinical relevance while mitigating risks associated with artificial intelligence (AI)-driven healthcare tools. The framework includes defining clinical goals, assembling a multidisciplinary team, data collection and preparation, model selection, fine-tuning, calibration, hallucination mitigation, user interface development, integration with electronic health records, real-world validation, and continuous improvement. Retrieval-augmented generation and fine-tuning approaches are evaluated for optimizing model adaptability. Bias detection, reinforcement learning from human feedback, and structured prompt engineering are incorporated to enhance reliability. Ethical and regulatory considerations, including the Health Insurance Portability and Accountability Act, General Data Protection Regulation, and AI-specific guidelines (DECIDE-AI, SPIRIT-AI, CONSORT-AI), are addressed to ensure responsible AI deployment. LLMs have the potential to enhance decision-making, research efficiency, and patient care in gastroenterology, but responsible deployment requires bias mitigation, transparency, and ongoing validation. Future research should focus on multi-institutional validation and AI-assisted clinical trials to establish LLMs as reliable tools in gastroenterology.
Keywords: artificial intelligence; framework; generative artificial intelligence; healthcare.
Plain language summary
How large language models could transform gastroenterology: a framework for future research and care Artificial intelligence (AI) is transforming healthcare by helping doctors make better decisions, analyze research faster, and improve patient care. Large language models (LLMs) are a type of AI that process and generate human-like text, making them useful in gastroenterology. This paper presents a structured framework for safely using LLMs in clinical practice, using Hepatitis C treatment as an example. The framework begins by setting clear goals, such as improving Hepatitis C treatment recommendations or making patient education easier to understand. A team of doctors, AI specialists, and data experts is assembled to ensure the model is medically accurate and practical. Next, relevant medical data from electronic health records (EHRs), clinical guidelines, and research studies is gathered and prepared to improve AI, ensuring it provides useful and fair recommendations. The right AI model is then chosen and improved to specialize in gastroenterology. To make sure the model is reliable and makes correct suggestions, its performance is checked and adjusted before use. A user-friendly interface is created so doctors can access AI-generated recommendations directly in EHRs and decision-support tools, making it easy to integrate into daily practice. Before full use, the AI is tested in real-world settings, where gastroenterologists review its recommendations for safety and accuracy. Once in use, ongoing updates based on doctor feedback help improve its performance. Ethical and legal safeguards, such as protecting patient privacy and ensuring fairness, guide its responsible use. Findings are then shared with the medical community, allowing for further testing and broader adoption. By following this framework, LLMs can help doctors make better decisions, personalize treatments, and improve efficiency, ultimately leading to better patient outcomes in gastroenterology.
© The Author(s), 2025.
Conflict of interest statement
P.B.: None. R.R.D: None. S.K.: Research grants from Rebiotix, Inc. (a Ferring company), Seres, Finch, Vedanta, and Pfizer; consulting fees from Takeda, Rise, and ProbioTech Inc., outside of the submitted work. The Associate Editor of Therapeutic Advances in Gastroenterology is the author of this paper; therefore, the peer review process was managed by alternative members of the Board and the submitting Editor was not involved in the decision-making process.
Figures

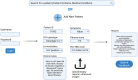
Similar articles
-
The Role of Large Language Models in Transforming Emergency Medicine: Scoping Review.JMIR Med Inform. 2024 May 10;12:e53787. doi: 10.2196/53787. JMIR Med Inform. 2024. PMID: 38728687 Free PMC article.
-
DeepSeek in Healthcare: Revealing Opportunities and Steering Challenges of a New Open-Source Artificial Intelligence Frontier.Cureus. 2025 Feb 18;17(2):e79221. doi: 10.7759/cureus.79221. eCollection 2025 Feb. Cureus. 2025. PMID: 39974299 Free PMC article.
-
Large Language Models and User Trust: Consequence of Self-Referential Learning Loop and the Deskilling of Health Care Professionals.J Med Internet Res. 2024 Apr 25;26:e56764. doi: 10.2196/56764. J Med Internet Res. 2024. PMID: 38662419 Free PMC article.
-
Exploring the benefits and challenges of AI-driven large language models in gastroenterology: Think out of the box.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2024 Nov;168(4):277-283. doi: 10.5507/bp.2024.027. Epub 2024 Sep 4. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2024. PMID: 39234774 Review.
-
Implications of Large Language Models for Quality and Efficiency of Neurologic Care: Emerging Issues in Neurology.Neurology. 2024 Jun 11;102(11):e209497. doi: 10.1212/WNL.0000000000209497. Epub 2024 May 17. Neurology. 2024. PMID: 38759131 Review.
References
-
- Sheikh H, Prins C, Schrijvers E. Artificial intelligence: definition and background. In: Sheikh H, Prins C, Schrijvers E. (eds) Mission AI: the new system technology. Cham: Springer International Publishing, 2023, pp. 15–41.
-
- Mitchell TM. Machine learning. New York: McGraw-Hill, 1997.
-
- Shai Shalev-Shwartz S, Ben-David S. Understanding machine learning. Cambridge: Cambridge University Press, 2014.
Publication types
LinkOut - more resources
Full Text Sources

