CAG-targeted brain-permeable therapy tested in biallelic humanized polyQ mouse models
- PMID: 40171277
- PMCID: PMC11960632
- DOI: 10.1016/j.omtn.2025.102496
CAG-targeted brain-permeable therapy tested in biallelic humanized polyQ mouse models
Abstract
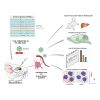
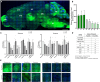
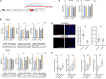
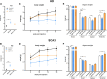
In polyglutamine (polyQ) diseases, including Huntington disease (HD) and spinocerebellar ataxia type 3 (SCA3), targeting the mutant CAG tract in mRNA could be a therapeutic strategy for lowering pathogenic protein. We explored the viability of this therapeutic strategy in vivo at the level of the reagent design, toxicity, systemic delivery, brain regions transduction, silencing efficiency, and allele preference. We designed a series of CAG-directed short hairpin RNAs (shRNAs) based on a previous A2 reagent, allele selective in vitro. Humanized HD (Hu128Q/21Q) and SCA3 (Ki150Q/21Q) mice with mutant ∼100 CAGs and normal 21 CAGs alleles were used to simulate biallelic conditions occurring in patients. We administered AAV-PHP.eB shRNAs-encoding vectors into the blood as an equivalent of non-invasive CAG-directed brain-targeted therapy crossing the blood-brain barrier. We demonstrate that optimized CAG-targeted A4(P10) and A4(P10,11) shReagents can lower mutant huntingtin and ataxin-3 protein and its aggregates by targeting brain regions selectively and with diminished toxicity compared to other tested shRNAs. The important considerations of the approach are the silencing efficiency depending on the transduction region and careful dose adjustment. Moreover, the CAG approach could be suitable to target somatic expansion. Our work paves the way toward developing the therapy for polyQ diseases, potentially shortening drug development.
Keywords: AAV-PHP.eB; CAG repeats targeting; Huntington disease; MT: Oligonucleotides: Therapies and Applications; SCA3; blood-brain barrier; gene therapy; neurodegenerative disease; shRNA; short hairpin RNA; spinocerebellar ataxia type 3; systemic delivery.
© 2025 The Authors. Published by Elsevier Inc. on behalf of The American Society of Gene and Cell Therapy.
Conflict of interest statement
A.F. is listed as a co-inventor on patents (US9970004B2 and US10329566B2) concerning the application of the RNAi approach in the treatment of diseases caused by the expansion of CAG trinucleotide repeats.
Figures


References
LinkOut - more resources
Full Text Sources

